锂有机电池中的磺酰胺基电解质可实现快速氧化还原动力学的准固态转换
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
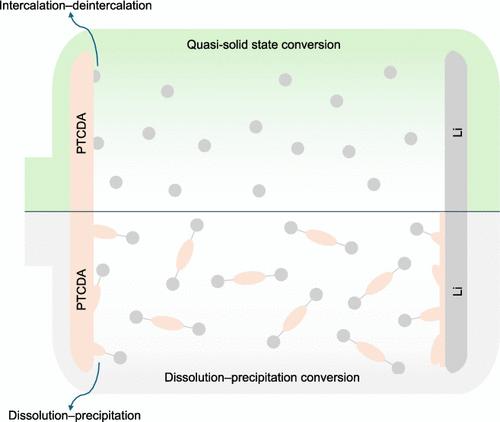
有机电极材料(如过烯-3,4,9,10-四羧酸二酐,PTCDA)在电解质中的严重溶解是一项重大挑战,不仅会降低其电化学性能,还会阻碍对基本氧化还原反应机制(包括内在动力学和溶剂共钙化)的解释。为了解决这些问题,我们提出了一种合理设计的磺酰胺基电解质,通过有效抑制 PTCDA 阴极在电解质中的溶解,实现 PTCDA 阴极的准固态转换(QSSC)。得益于 QSSC,锂||PTCDA 电池在循环 300 次后仍能保持原始容量的 95.8%,能量效率高且稳定,甚至可与层状过渡金属氧化物阴极媲美,大大优于 PTCDA 溶解度较高的醚基电解质。高能效表明 PTCDA 的 QSSC 具有内在的快速氧化还原动力学。此外,还通过密度泛函理论/分子动力学计算研究了溶剂共钙化机制。这项研究为设计高稳定、高效的有机锂电池电解质提供了一种策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quasi-Solid-State Conversion with Fast Redox Kinetics Enabled by a Sulfonamide-Based Electrolyte in Li–Organic Batteries
The serious dissolution of organic electrode materials (e.g., perylene-3,4,9,10-tetracarboxylic dianhydride, PTCDA) in electrolytes is a major challenge, deteriorating their electrochemical performances and hindering the interpretation of the fundamental redox reaction mechanisms including the intrinsic kinetics and the solvent cointercalation. To address these issues, we propose a rationally designed sulfonamide-based electrolyte to enable a quasi-solid-state conversion (QSSC) of the PTCDA cathode by effectively suppressing its dissolution in the electrolyte. Benefiting from the QSSC, the Li||PTCDA cells can retain ∼95.8% of the original capacity after 300 cycles with both high and stable energy efficiencies >95%, even comparable to the layered transition-metal oxide cathodes, greatly outperforming an ether-based electrolyte with a high PTCDA solubility. The high energy efficiencies indicate that the QSSC of PTCDA has intrinsic fast redox kinetics. Furthermore, the solvent cointercalation mechanism was investigated by density functional theory/molecular dynamic calculations. This work develops a strategy for designing electrolytes for highly stable and efficient Li–organic batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


