镍催化乙烯基氯硅烷与轴向手性双芳基亲电体的立体特异性还原交叉偶联反应
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对映体有机硅烷是材料科学和有机合成中重要的手性分子。轴向手性有机硅烷的合成在应用方面尤为重要。在此,我们报告了一种镍催化的乙烯基氯硅烷与立体受阻手性双芳基亲电体的还原交叉亲电偶联,从而合成异构双芳基有机硅烷。在温和的条件下,可以高效地获得各种对映体轴向手性乙烯基硅烷。展示了新的手性含硅烯配体的合成转化和应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
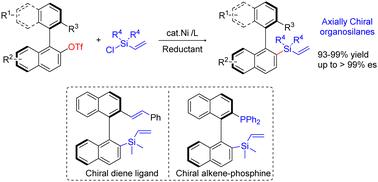
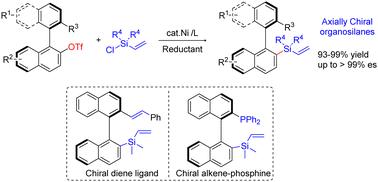
Nickel-catalyzed stereospecific reductive cross-coupling of vinyl chlorosilanes with axially chiral biaryl electrophiles†
Enantioenriched organosilanes are important chiral molecules in materials science and organic synthesis. The synthesis of axially chiral organosilanes is particularly significant in terms of applications. Herein, we report a Ni-catalyzed reductive cross-electrophile coupling of vinyl chlorosilanes with sterically hindered chiral biaryl electrophiles for the synthesis of atropisomeric biaryl organosilanes. Various enantioenriched axially chiral vinylsilanes are accessible in high efficiency under mild conditions. The synthetic transformations and applications of new chiral silicon-containing alkene ligands are demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


