微生物诱导的巨噬细胞犬尿氨酸代谢改变促使克罗恩病中爬行脂肪的形成
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
克罗恩病肠系膜组织的增生被称为爬行脂肪(CrF),与手术复发有关。虽然在 CrF 中发现了微生物群易位和定植,但令人信服的小鼠表型和 CrF 形成的潜在机制仍不清楚。利用对 CrF 和不同小鼠模型的单核 RNA(snRNA)测序,我们证明了肠道共生菌 Achromobacter pulmonis 可通过改变巨噬细胞诱导肠系膜脂肪生成。靶向代谢组分析表明,L-犬尿氨酸是 CrF 中含量最高的代谢物。吲哚胺 2,3-二氧合酶 1(IDO1)的上调增强了犬尿氨酸的代谢,并推动了肠系膜脂肪的生成。通过对小鼠肠系膜组织和巨噬细胞特异性 IDO1 基因敲除小鼠进行单细胞 RNA(scRNA)测序,我们验证了巨噬细胞来源的 L-犬尿氨酸在肠系膜脂肪生成中的作用。从机理上讲,L-犬尿氨酸诱导的脂肪生成是由脂肪细胞中的芳基烃受体介导的。服用 IDO1 抑制剂或降解 L-犬尿氨酸的细菌可减轻小鼠肠系膜脂肪的生成。总之,我们的研究表明,微生物诱导的巨噬细胞新陈代谢调节可促进 CrF 的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
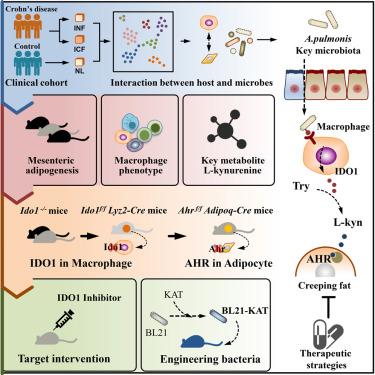
Microbiota-induced alteration of kynurenine metabolism in macrophages drives formation of creeping fat in Crohn’s disease
Hyperplasia of mesenteric tissues in Crohn’s disease, called creeping fat (CrF), is associated with surgical recurrence. Although microbiota translocation and colonization have been found in CrF, convincing mouse phenotypes and the underlying mechanisms of CrF formation remain unclear. Utilizing single-nucleus RNA (snRNA) sequencing of CrF and different mouse models, we demonstrate that the commensal Achromobacter pulmonis induces mesenteric adipogenesis through macrophage alteration. Targeted metabolome analysis reveals that L-kynurenine is the most enriched metabolite in CrF. Upregulation of indoleamine 2,3-dioxygenase 1 (IDO1) enhances kynurenine metabolism and drives mesenteric adipogenesis. Leveraging single-cell RNA (scRNA) sequencing of mouse mesenteric tissues and macrophage-specific IDO1 knockout mice, we verify the role of macrophage-sourced L-kynurenine in mesenteric adipogenesis. Mechanistically, L-kynurenine-induced adipogenesis is mediated by the aryl hydrocarbon receptors in adipocytes. Administration of an IDO1 inhibitor or bacteria engineered to degrade L-kynurenine alleviates mesenteric adipogenesis in mice. Collectively, our study demonstrates that microbiota-induced modulation of macrophage metabolism potentiates CrF formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


