金属转运体 SMF11 和铁还原酶 FRE1 在白色念珠菌铁平衡中的共同作用
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
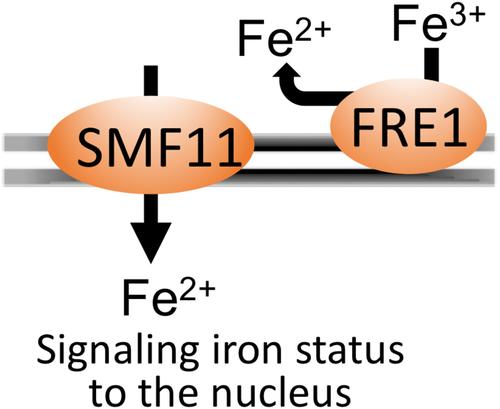
致病真菌必须适当地感知宿主体内是否存在铁等必需金属。在白色念珠菌和其他酵母菌中,对 Fe 的感知涉及线粒体中的 Fe-S 簇。酵母的 Fe-S 簇组装突变体即使在铁元素丰富的情况下也能感知到铁元素的限制,并过度积累铁元素。我们在白僵菌 SMF11(一种二价金属的 NRAMP 转运体)的突变体中也观察到了这种对铁的感应紊乱。smf11突变体同时过度积累锰和铁,而锰的升高是铁超载的次要原因。与 Fe-S 生物发生突变体一样,smf11∆/∆ 突变体也会出现铁还原酶的上调,而铁还原酶在高铁元素条件下通常会被抑制,铁的输入也会被激活。然而,与 Fe-S 生物发生突变体不同,smf11∆/∆ 突变体的线粒体 Fe-S 酶没有缺陷。耐人寻味的是,白僵菌中编码铁还原酶的 fre1∆/∆ 突变体也会出现这种情况,即铁元素感应紊乱,但不抑制 Fe-S 簇。fre1 和 smf11 的突变体在细胞壁、丝状化和形态发生的 ROS 爆发(一个依赖铁的过程)中表现出类似的扰动。与 FRE1 一样,SMF11 对小鼠播散性念珠菌病模型中的毒力也很重要。我们提出了一个模型,在该模型中,FRE1 和 SMF11 在线粒体 Fe-S 通路之外运作,为铁感应提供亚铁。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Converging Roles of the Metal Transporter SMF11 and the Ferric Reductase FRE1 in Iron Homeostasis of Candida albicans
Pathogenic fungi must appropriately sense the host availability of essential metals such as Fe. In Candida albicans and other yeasts, sensing of Fe involves mitochondrial Fe-S clusters. Yeast mutants for Fe-S cluster assembly sense Fe limitation even when Fe is abundant and hyperaccumulate Fe. We observe this same disrupted Fe sensing with C. albicans mutants of SMF11, a NRAMP transporter of divalent metals. Mutants of smf11 hyperaccumulate both Mn and Fe and the elevated Mn is secondary to Fe overload. As with Fe-S biogenesis mutants, smf11∆/∆ mutants show upregulation of ferric reductases that are normally repressed under high Fe, and Fe import is activated. However, unlike Fe-S biogenesis mutants, smf11∆/∆ mutants show no defects in mitochondrial Fe-S enzymes. Intriguingly, this exact condition of disrupted Fe sensing without inhibiting Fe-S clusters occurs with C. albicans fre1∆/∆ mutants encoding a ferric reductase. Mutants of fre1 and smf11 display similar perturbations in the cell wall, in filamentation and in the ROS burst of morphogenesis, a Fe-dependent process. As with FRE1, SMF11 is important for virulence in a mouse model for disseminated candidiasis. We propose a model in which FRE1 and SMF11 operate outside the mitochondrial Fe-S pathway to donate ferrous Fe for Fe sensing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


