苄基 C-H 键的正式对映选择性羟基化和氨基化的化学酶级联反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报告了一种人工过氧酶(CoN4SA-POase)的合成和表征,这种人工过氧酶通过在聚合碳氮上支持单原子钴而具有 CoN4 活性位点,在芳香烃氧化成酮的过程中表现出高活性、高选择性、高稳定性和可重复使用性。密度泛函理论计算揭示了人工过氧酶与天然过氧酶不同的催化机理。此外,利用连续流系统将 CoN4SA-POase 与对映体互补酮还原酶以及胺脱氢酶结合起来,实现了从碳氢化合物对映体选择性合成手性醇和胺,大大提高了生产率。这项工作仿效自然并超越自然,为基于血红素酶的转化提供了一种前景广阔的设计理念。本文章由计算机程序翻译,如有差异,请以英文原文为准。
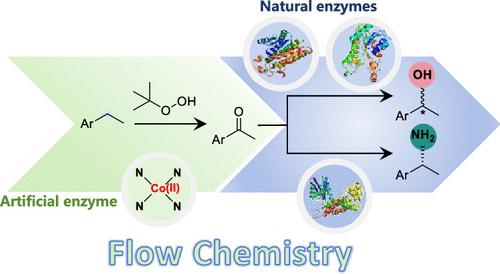
A Chemoenzymatic Cascade for the Formal Enantioselective Hydroxylation and Amination of Benzylic C–H Bonds
We report the synthesis and characterization of an artificial peroxygenase (CoN4SA-POase) with CoN4 active sites by supporting single-atom cobalt on polymeric carbon nitrogen, which exhibits high activity, selectivity, stability, and reusability in the oxidation of aromatic alkanes to ketones. Density functional theory calculations reveal a different catalytic mechanism for the artificial peroxygenase from that of natural peroxygenases. In addition, continuous-flow systems are employed to combine CoN4SA-POase with enantiocomplementary ketoreductases as well as an amine dehydrogenase, enabling the enantioselective synthesis of chiral alcohols and amines from hydrocarbons with significantly improved productivity. This work, emulating nature and beyond nature, provides a promising design concept for heme enzyme-based transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


