作为能量代谢调节器的三维半导体网络驱动骨再生中的血管生成
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
血管化不足是骨植入失败的主要原因。能量代谢的管理是实现血管化骨结合的关键。根据骨半导体特性和半导体异质结的电特性,我们设计了一种三维半导体异质结网络(3D-NTBH)植入体,旨在调节细胞能量代谢,从而推动血管生成,促进骨再生。三维异质结界面可促进电子转移,并在纳米级界面上建立内部电场。研究发现,3D-NTBH 能明显加速内皮细胞的糖酵解,从而迅速提供能量支持细胞代谢活动,最终推动骨组织内的血管生成。分子动力学模拟表明,3D-NTBH 可促进纤维连接蛋白的 Arg-Gly-Asp 肽结合位点的暴露,从而调节内皮细胞的糖酵解。进一步的证据表明,3D-NTBH 可促进体内血管网络的早期重建和骨再生。这项研究成果为血管化植入物的设计提供了一个前景广阔的研究视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
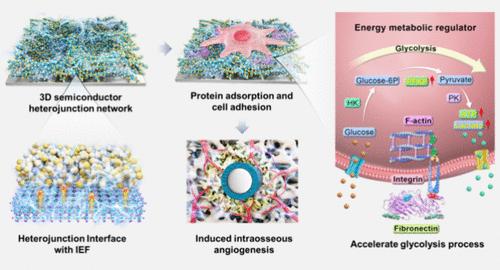
Three-Dimensional Semiconductor Network as Regulators of Energy Metabolism Drives Angiogenesis in Bone Regeneration
Insufficient vascularization is a primary cause of bone implantation failure. The management of energy metabolism is crucial for the achievement of vascularized osseointegration. In light of the bone semiconductor property and the electric property of semiconductor heterojunctions, a three-dimensional semiconductor heterojunction network (3D-NTBH) implant has been devised with the objective of regulating cellular energy metabolism, thereby driving angiogenesis for bone regeneration. The three-dimensional heterojunction interfaces facilitate electron transfer and establish internal electric fields at the nanoscale interfaces. The 3D-NTBH was found to noticeably accelerate glycolysis in endothelial cells, thereby rapidly providing energy to support cellular metabolic activities and ultimately driving angiogenesis within the bone tissue. Molecular dynamic simulations have demonstrated that the 3D-NTBH facilitates the exposure of fibronectin’s Arg-Gly-Asp peptide binding site, thereby regulating the glycolysis of endothelial cells. Further evidence suggests that 3D-NTBH promotes early vascular network reconstruction and bone regeneration in vivo. The findings of this research offer a promising research perspective for the design of vascularizing implants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


