脂质载脂蛋白-受体相互作用的减少可防止载脂蛋白及其脂质货物在溶酶体中致病
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
虽然载脂蛋白 E(APOE)是晚发性阿尔茨海默病(LOAD)最强的遗传修饰因子,但异构体依赖性风险的分子机制以及载脂蛋白 E 相关脂质的相关性仍然难以捉摸。在这里,我们报告了脂质化载脂蛋白 E2(lipApoE2)与低密度脂蛋白(LDL)受体(LDLR)结合受损的情况,这避免了在脂质载脂蛋白 E3/E4 中观察到的 LDLR 循环缺陷,并减少了胆固醇酯(CE)的吸收,而胆固醇酯是与神经变性相关的脂质。在人类神经元中,加入携带多不饱和脂肪酸(PUFAs)-CE 的载脂蛋白E,发现了与脂褐素沉着病(一种由脂质过氧化引起的与年龄有关的溶酶体病理学)相关的等位基因系列(载脂蛋白E4 >;载脂蛋白E3 >;载脂蛋白E2)。脂褐质增加了溶酶体中 tau 纤维的积累,并在 APOE4 小鼠大脑中升高,tau 病理学加剧了这种升高。在野生型小鼠的海马内注射 PUFA-CE-lipApoE4 足以诱发脂褐素沉着症。最后,保护性的克赖斯特彻奇突变也减少了 LDLR 的结合,并表现为 ApoE2。总之,我们的数据有力地表明,脂载脂蛋白-LDLR相互作用的减少通过降低脂载脂蛋白和相关致病脂质在溶酶体内靶向的有害影响,将 LOAD 风险降至最低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
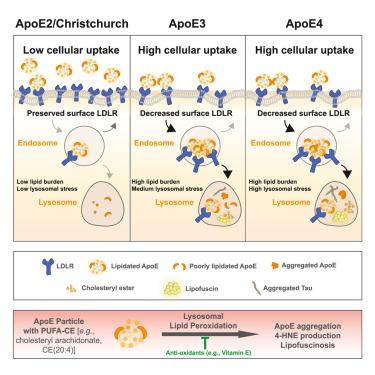
Decreased lipidated ApoE-receptor interactions confer protection against pathogenicity of ApoE and its lipid cargoes in lysosomes
While apolipoprotein E (APOE) is the strongest genetic modifier for late-onset Alzheimer’s disease (LOAD), the molecular mechanisms underlying isoform-dependent risk and the relevance of ApoE-associated lipids remain elusive. Here, we report that impaired low-density lipoprotein (LDL) receptor (LDLR) binding of lipidated ApoE2 (lipApoE2) avoids LDLR recycling defects observed with lipApoE3/E4 and decreases the uptake of cholesteryl esters (CEs), which are lipids linked to neurodegeneration. In human neurons, the addition of ApoE carrying polyunsaturated fatty acids (PUFAs)-CE revealed an allelic series (ApoE4 > ApoE3 > ApoE2) associated with lipofuscinosis, an age-related lysosomal pathology resulting from lipid peroxidation. Lipofuscin increased lysosomal accumulation of tau fibrils and was elevated in the APOE4 mouse brain with exacerbation by tau pathology. Intrahippocampal injection of PUFA-CE-lipApoE4 was sufficient to induce lipofuscinosis in wild-type mice. Finally, the protective Christchurch mutation also reduced LDLR binding and phenocopied ApoE2. Collectively, our data strongly suggest decreased lipApoE-LDLR interactions minimize LOAD risk by reducing the deleterious effects of endolysosomal targeting of ApoE and associated pathogenic lipids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


