分子全息图的时空建模
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
量化胚胎发生过程中的时空动态对于了解先天性疾病至关重要。我们开发了三维时空建模框架 Spateo (https://github.com/aristoteo/spateo-release),并将其应用于 E9.5 和 E11.5 年龄段的三维小鼠胚胎发生图谱,捕获了 800 万个细胞。Spateo 实现了可扩展、局部、非刚性配准、多切片细化和网格校正,以创建整个胚胎的分子全息图。它引入了数字化方法来揭示从亚细胞到整个器官的多层次生物学,沿着出现的三维结构(如中脑-后脑边界(MHB)等次级组织者)的正交轴识别表达梯度。Spateo 还进一步联合建立了细胞间和细胞内相互作用模型,以剖析三维结构中的信号景观,包括内侧限区(ZLI)。最后,Spateo 引入了细胞迁移的 "形态计量矢量场",并结合空间微分几何揭示了非对称小鼠心脏器官发生和其他方面的分子程序,将宏观变化与分子动力学联系起来。因此,Spateo 能够在三维空间中从分子水平研究器官生态学。本文章由计算机程序翻译,如有差异,请以英文原文为准。
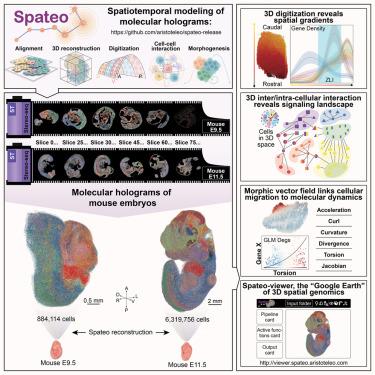
Spatiotemporal modeling of molecular holograms
Quantifying spatiotemporal dynamics during embryogenesis is crucial for understanding congenital diseases. We developed Spateo (https://github.com/aristoteo/spateo-release), a 3D spatiotemporal modeling framework, and applied it to a 3D mouse embryogenesis atlas at E9.5 and E11.5, capturing eight million cells. Spateo enables scalable, partial, non-rigid alignment, multi-slice refinement, and mesh correction to create molecular holograms of whole embryos. It introduces digitization methods to uncover multi-level biology from subcellular to whole organ, identifying expression gradients along orthogonal axes of emergent 3D structures, e.g., secondary organizers such as midbrain-hindbrain boundary (MHB). Spateo further jointly models intercellular and intracellular interaction to dissect signaling landscapes in 3D structures, including the zona limitans intrathalamica (ZLI). Lastly, Spateo introduces “morphometric vector fields” of cell migration and integrates spatial differential geometry to unveil molecular programs underlying asymmetrical murine heart organogenesis and others, bridging macroscopic changes with molecular dynamics. Thus, Spateo enables the study of organ ecology at a molecular level in 3D space over time.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


