AMn2O4(A = Ni、Co、Cu)氧载体对苯的化学循环重整:通过实验和 DFT 研究分析活性氧的迁移途径
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
化学循环重整(CLR)为生物质焦油的清洁高效利用提供了一种新的解决方案。氧载体(OC)的多功能性对提高重整效率至关重要。以苯为焦油模型化合物,研究了锰基尖晶石 OC(AMn2O4,A = Ni、Co、Cu)在 CLR 工艺中的特性。详细的表征和实验结果表明,锰基尖晶石具有出色的结构稳定性。NiMn2O4 对苯的转化效果最为显著,在 850 ℃、S/C = 1.0 和 WHSV = 3.0 h-1 条件下转化率最高,达到 95.77 %。经过 40 个循环后,NiMn2O4 和 CoMn2O4 在苯重整方面保持了显著的催化活性,在最后一个循环中的转化率分别达到 92.96 % 和 90.07 %。密度泛函理论(DFT)计算表明,加入 H2O 会提高 NiMn2O4 的活性。与单独吸附苯相比,加入 H2O 后吸附能从 -2.20 eV 下降到 -2.54 eV。直接证明了 NiMn2O4 (100) 活性氧在 H2O 存在或不存在时的迁移路径。在没有 H2O 的情况下,NiMn2O4 晶格氧直接氧化 C6H5* 的活化能势垒占优势(0.98 eV),但 H2O 解离产生的 OH* 表现出很高的活性,氧化 C6H5* 生成关键中间产物 C6H5O* 的活化能势垒仅为 0.35 eV。此外,H2O 在氧空位的补充中起着主导作用。氧迁移机制的阐明为设计催化氧化的高效 OC 提供了新的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
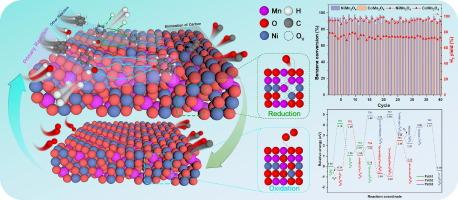
AMn2O4 (A = Ni, Co, Cu) oxygen carrier chemical looping reforming of benzene: Migration pathways of reactive oxygen species by experimental and DFT investigations
Chemical looping reforming (CLR) provides a novel solution for clean and efficient utilization of biomass tar. The versatility of oxygen carrier (OC) is essential for improving reforming efficiency. The properties of Mn-based spinel OCs (AMn2O4, A = Ni, Co, Cu) were investigated in the CLR process using benzene as a tar model compound. Detailed characterization and experimental results demonstrate the excellent structural stability of Mn-based spinel. The NiMn2O4 showed the most prominent reforming effect on benzene with the highest conversion of 95.77 % at 850 °C, S/C = 1.0, and WHSV = 3.0 h−1. After 40 cycles, NiMn2O4 and CoMn2O4 maintained significant catalytic activity for benzene reforming, achieving conversions of 92.96 % and 90.07 %, respectively, in the final cycle. Density functional theory (DFT) calculations demonstrate that the addition of H2O increases the activity of NiMn2O4. Compared to benzene adsorption alone, the adsorption energy decreased from −2.20 eV to −2.54 eV after the addition of H2O. The migration path of NiMn2O4 (100) reactive oxygen species in the presence or absence of H2O is directly demonstrated. In the absence of H2O, the activation energy barrier for direct oxidation of C6H5* by NiMn2O4 lattice oxygen is dominant (0.98 eV), but OH* produced by dissociation of H2O exhibits high activity, and oxidation of C6H5* to produce the key intermediate product C6H5O* has an activation energy barrier of only 0.35 eV. In addition, H2O has a predominant role in the replenishment of oxygen vacancies. The elucidation of the oxygen migration mechanism provides new guidance for the design of efficient OCs for catalytic oxidation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


