一种含{Ru(C6H6)}的异聚钼酸盐,具有良好的胺氧化偶联光催化活性和质子传导性能
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
我们通过一锅反应制备了一种非典型的含{Ru(C6H6)}的异多钼酸盐 Cs8K2Na2H4{[(C6H6)Ru]6Mo24O86}-35H2O (1)。化合物 1 的晶体学表征显示,它含有一个不同寻常的{Mo24O86}簇和六个{Ru(C6H6)}基团,是含{Ru(C6H6)}多氧金属盐领域中{Ru(C6H6)}基团数量最多的化合物。结果表明,化合物 1 在 90 °C 和 95% 相对湿度条件下显示出优异的质子传导性(9.8 × 10-3 S cm-1)。根据活化能大于 0.4 eV(1.47 eV)的计算结果,可以确定化合物 1 的质子传导过程符合 Vehicle 机理。更有趣的是,化合物 1 被用作催化剂实现了胺的异相氧化偶联,并在常温、可见光照射(400 nm)和氧气环境下实现了对 N-亚苄基苄胺的高产率(98.9%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
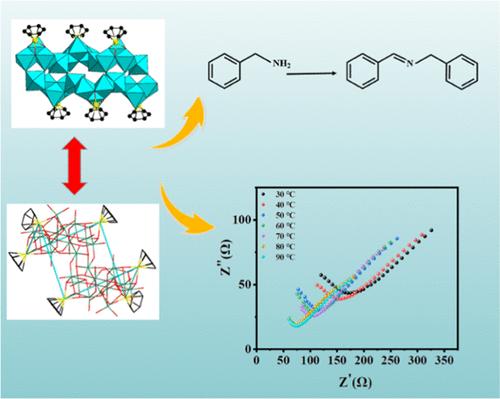
A {Ru(C6H6)}-Containing Isopolymolybdate with Good Photocatalytic Activity for Oxidative Coupling of Amines, and Proton Conduction Properties
We present a nonclassical {Ru(C6H6)}-containing isopolymolybdate Cs8K2Na2H4{[(C6H6)Ru]6Mo24O86}·35H2O (1) via one-pot reaction. The crystallographic characterization of compound 1 revealed that it contains an unusual {Mo24O86} cluster together with six {Ru(C6H6)} groups, having the largest number of {Ru(C6H6)} groups within the field of {Ru(C6H6)}-containing polyoxometalates. Results showed that compound 1 displayed excellent proton conductivity (9.8 × 10–3 S cm–1) at 90 °C and 95% relative humidity. Based on the calculation result of activation energy, which was more than 0.4 eV (1.47 eV), it was determined that the proton conduction process for compound 1 was in accordance with the Vehicle mechanism. More interestingly, compound 1 was exploited as a catalyst to achieve the heterogeneous oxidative coupling of amines and realized a high yield (98.9%) toward N-benzylidenebenzylamine under ambient temperature and visible light irradiation (>400 nm) and O2 atmosphere.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


