S 型银/银硼/硼异质结光催化剂的高效制备及其对降解多菌灵的影响:机理、途径和毒理学
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
多菌灵(CBZ)作为一种高效苯并咪唑类杀菌剂,对各种作物上的真菌具有良好的防治效果。然而,在水、大气、土壤和作物中过量使用 CBZ 会产生严重影响。高效降解 CBZ 是降低其毒性的有效途径。本研究采用简单的一步溶热原位法有效制备了 S 型结构的 Ag/AgBr/BiOBr 异质结光催化剂,并首次应用于 CBZ 的矿化降解。全面研究了AgBr与BiOBr的摩尔比、催化剂用量、CBZ浓度、原溶液pH值和无机盐离子对CBZ光催化降解性能的影响。结果表明,在可见光照射下,0.9-Ag/AgBr/BiOBr(0.9-AAB)对浓度为 10 mg/L 的 CBZ(原液 pH 值为 10)的光催化降解性能最好(88.9%)。90 分钟后,降解效率达到 86.0%,对应的 TOC 去除效率为 84.0%。结果表明,根据自由基淬灭实验和电子自旋共振谱,主要的活性物种是 1O2 和 -O2- 自由基。结合 XPS 表征结果,深入揭示了 S 型异质结的电子传递机理。此外,通过中间体鉴定和 DFT 福井指数的理论计算,提出了 CBZ 的降解途径。最后,根据T.E.S.T.预测了CBZ的毒性和降解中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
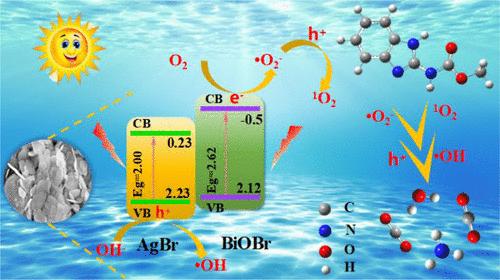
Efficient Preparation of S-Scheme Ag/AgBr/BiOBr Heterojunction Photocatalysts and Implications for Degradation of Carbendazim: Mechanism, Pathway, and Toxicology
Carbendazim (CBZ), as a highly effective benzimidazole fungicide, has a good control effect on various crops caused by fungi. However, excessive use of CBZ in water, atmosphere, soil, and crops has serious effects. The efficient degradation of CBZ is an effective way to reduce its toxic effect. In this work, the type of S-scheme Ag/AgBr/BiOBr heterojunction photocatalyst was effectively prepared by a simple one-step solvothermal in situ method and first applied to the mineralization and degradation of CBZ. The effects of the molar ratio of AgBr to BiOBr, catalyst dosage, CBZ concentration, pH value of the original solution, and inorganic salt ions on the photocatalytic degradation performance of CBZ were comprehensively studied. The results showed that, under visible light irradiation, 0.9-Ag/AgBr/BiOBr (0.9-AAB) exhibited the best photocatalytic degradation performance (88.9%) against the concentration at 10 mg/L of CBZ in original solutions with pH of 10. However, the degradation effect was also good at pH 7. After 90 min, the degradation efficiency reached 86.0%, corresponding to a TOC removal efficiency of 84.0%. The results indicate that the main active species are 1O2 and •O2– free radicals according to the free radical quenching experiments and electron spin resonance spectra. Combined with the XPS characterization results, the electron transfer mechanism of the S-scheme heterojunction was deeply revealed. Additionally, the degradation pathway of CBZ was proposed through both the intermediate identification and the theoretical calculation derived from the DFT Fukui index. Finally, the toxicity of CBZ and the degradation intermediates were predicted based on the T.E.S.T.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


