合成原始沸石 Na-A 并评估其从模拟低放射性废物溶液中去除 ReO4- 离子(99TcO4- 的替代物)的萃取效率
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以高岭土为经济可行的前驱体,通过水热法、碱熔法和声化学法合成了沸石 Na-A。利用 XRD、傅立叶变换红外光谱、扫描电镜和拉曼光谱对合成的 Na-A 沸石样品进行了表征。使用 BET 比表面积分析仪进行了比表面积和孔径分布分析(采用 BJH 和 DFT 模型)。此外,还利用 TG-DTA 进行了热降解研究,以检测沸石 Na-A 在高温下的热稳定性。此外,还利用高岭土衍生的沸石 Na-A 对溶液介质进行了原位改性,首次将其用于萃取过铼酸根离子(ReO4-),后者是过硫酸根离子(99TcO4-)的非放射性替代物,无需进行任何后期合成改性(使用有毒的表面活性剂等)。声化学合成的沸石 Na-A 在从模拟低浓度废物溶液中固相萃取 ReO4- 离子时表现出卓越的吸附性能。研究发现,吸附过程遵循伪二阶动力学。Langmuir 等温线模型与实验数据完全吻合(R2 = 0.997),在 pH ∼ 11 时的最大吸附容量为 926.8 mg/g,与之前报道的众多材料相比,吸附能力更强。XPS 证实萃取的铼为 NH4Re(VII)O4,为吸附机制提供了重要见解,并验证了声化学合成沸石对 ReO4 吸附的适用性。此外,对吸附了 ReO4- 的沸石 Na-A 进行的拉曼研究表明,没有特征性呼吸带,这表明由于孔隙中吸附分子的占据,孔隙开口已经关闭。这项研究不仅强调了利用声化学合成的沸石 Na-A 作为一种高效、良性和具有成本效益的吸附剂来去除核废料中的 99TcO4-,还强调了它在其他各种工业过程中的潜在可持续应用,如废水处理、催化、气体分离、污染控制、从工业废水中回收资源以及在制药业中选择性去除离子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
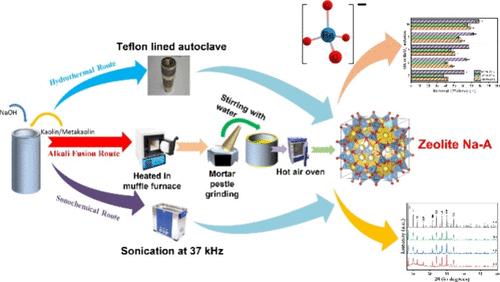
Synthesis and Evaluation of the Extraction Efficiency of Pristine Zeolite Na-A to Remove ReO4– Ions (Surrogate of 99TcO4–) from a Simulated Low-Level Waste Solution
Zeolite Na-A was synthesized through hydrothermal, alkali-fusion, and sonochemical methods, using kaolin as an economically viable precursor. The synthesized zeolite Na-A samples were characterized using XRD, FT-IR, SEM, and Raman spectroscopy. Specific surface area and pore size distribution analyses (by BJH and DFT models) were conducted using a BET surface area analyzer. Additionally, thermal degradation studies were performed by TG-DTA to check the thermal stability of zeolite Na-A at high temperatures. Furthermore, kaolin-derived zeolite Na-A was employed for the extraction of perrhenate ions (ReO4–), which are a nonradioactive surrogate for pertechnetate ions (99TcO4–) without any postsynthetic modifications (using toxic surfactants, etc.) utilizing in situ modification of the solution medium for the first time. The sonochemically synthesized zeolite Na-A demonstrated superior sorption performance for the solid-phase extraction of ReO4– ions from the simulated low-level waste solution. The adsorption process was found to follow pseudo-second-order kinetics. The Langmuir isotherm model fit perfectly with the experimental data (R2 = 0.997) and exhibited a maximum sorption capacity of 926.8 mg/g at pH ∼11, showing superior sorption capacities compared to those of the numerous materials reported earlier. XPS confirmed the speciation of extracted rhenium as NH4Re(VII)O4, providing critical insights into the adsorption mechanisms and validating the suitability of the sonochemically synthesized zeolites toward ReO4– sorption. Furthermore, Raman studies of ReO4– adsorbed zeolite Na-A reflect the absence of characteristic breathing bands, indicating the closure of the pore openings due to the occupancy of adsorbate moieties within the pores. This study not only highlights the utilization of sonochemically synthesized zeolite Na-A as an efficient, benign, and cost-effective adsorbent for 99TcO4– removal from nuclear waste but also emphasizes its potential sustainable applications in various other industrial processes such as wastewater treatment, catalysis, gas separation, pollution control, and resource recovery from industrial effluents and in the pharmaceutical industry for selective ion removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


