矿井溪流沉积物中光敏溶解有机物驱动的新型砷酸盐光电子辅助微生物还原作用
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
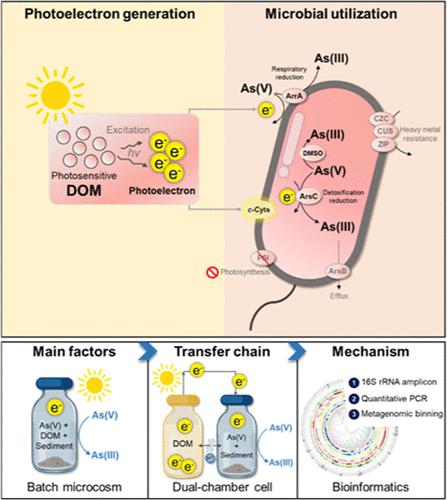
砷酸盐(As(V))的微生物还原是矿井溪流沉积物中砷迁移的重要原因,主要由利用溶解有机物(DOM)作为碳源的异养微生物驱动。这项研究揭示了沉积物中的一种新型还原途径,即光敏 DOM 产生光电子,刺激多种非光养性微生物还原 As(V)。这种微生物光致发光还原 As(V)(PEAsR)是利用微生态培养法进行研究的,结果表明光电子从 DOM 转移到本地沉积物微生物,从而使微生物还原 As(V)的速率提高了 50%。两个还原 As(V)的标记基因 arrA 和 arsC 的丰度大幅增加,证实了 PEAsR 的微生物性质,而不是光电化学过程。光电子离子不太可能刺激光自养生长。相反,不同的非光养菌属,如 Cupriavidus、Sphingopyxis、Mycobacterium 和 Bradyrhizobium,跨越 13 个目,富集了 10-50 倍。元基因组分选揭示了它们介导光电子辅助还原 As(V)的遗传潜力。这些微生物含有参与呼吸还原 As(V)、解毒还原 As(V)、二甲亚砜还原酶家族、c 型细胞色素和多种重金属抗性的重要基因,但缺乏完整的光合作用系统。新型微生物 PEAsR 途径为光电子利用和非光养性 As(V)还原微生物之间的相互作用提供了新的见解,这可能对矿山溪流沉积物中砷污染的迁移产生深远影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel Photoelectron-Assisted Microbial Reduction of Arsenate Driven by Photosensitive Dissolved Organic Matter in Mine Stream Sediments
The microbial reduction of arsenate (As(V)) significantly contributes to arsenic migration in mine stream sediment, primarily driven by heterotrophic microorganisms using dissolved organic matter (DOM) as a carbon source. This study reveals a novel reduction pathway in sediments that photosensitive DOM generates photoelectrons to stimulate diverse nonphototrophic microorganisms to reduce As(V). This microbial photoelectrophic As(V) reduction (PEAsR) was investigated using microcosm incubation, which showed the transfer of photoelectrons from DOM to indigenous sediment microorganisms, thereby leading to a 50% higher microbial reduction rate of As(V). The abundance of two marker genes for As(V) reduction, arrA and arsC, increased substantially, confirming the microbial nature of PEAsR rather than a photoelectrochemical process. Photoelectron ion is unlikely to stimulate photolithoautotrophic growth. Instead, diverse nonphototrophic genera, e.g., Cupriavidus, Sphingopyxis, Mycobacterium, and Bradyrhizobium, spanning 13 orders became enriched by 10–50 folds. Metagenomic binning revealed their genetic potential to mediate the photoelectron-assisted reduction of As(V). These microorganisms contain essential genes involved in respiratory As(V) reduction, detoxification As(V) reduction, dimethyl sulfoxide reductase family, c-type cytochromes, and multiple heavy-metal resistance but lack a complete photosynthesis system. The novel microbial PEAsR pathway offers new insights into the interaction between photoelectron utilization and nonphototrophic As(V)-reducing microorganisms, which may have profound implications for arsenic pollution transportation in mine stream sediment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


