以血管紧张素 II 受体为靶标的荧光配体的合成和药理特征,这些荧光配体来自于激动剂、β-阿司匹林类激动剂和拮抗剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
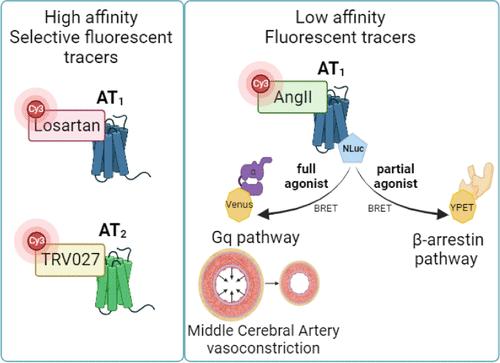
血管紧张素 II(AngII)调节脑循环,与 AT1 和 AT2 受体的结合亲和力相似。TRV027等偏性AT1激动剂能够选择性地激活β-arrestin,同时阻断Gq通路,因此有望成为新的治疗药物。我们需要新的药理学工具来进一步探索偏性 AT1 激动剂对细胞或组织(如脑血管)的影响。我们设计并合成了基于 AngII、TRV027 或 AT1 拮抗剂洛沙坦的新型荧光衍生物。我们对它们进行了药理学表征,以确定它们的选择性、效力以及激活或不激活细胞和脑动脉中特定 AT1 转导通路的能力。我们报告了首个基于洛沙坦的高度 AT1 选择性荧光配体,该配体保持了高亲和力的拮抗剂活性。TRV027 的荧光衍生物具有 AT2 选择性,并失去了 AT1 β-阻遏素的偏向性。这些新配体可用于体外和体内追踪 AT1 或 AT2 受体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and Pharmacological Characterization of Fluorescent Ligands Targeting the Angiotensin II Receptors Derived from Agonists, β-Arrestin-Biased Agonists, and Antagonists
Angiotensin II (AngII) regulates cerebral circulation and binds with a similar affinity to AT1 and AT2 receptors. Biased AT1 agonists, such as TRV027, which are able to selectively activate β-arrestin while blocking the Gq pathway, appear promising as new therapeutics. New pharmacological tools are needed to further explore the impact of biased AT1 agonists on cells or tissues, such as the cerebral vessels. We designed and synthesized new fluorescent derivatives based on AngII, TRV027, or the AT1 antagonist losartan. We conducted pharmacological characterization to determine their selectivity, potency, and ability to activate or not specific AT1 transduction pathways in cells and cerebral arteries. We report the first highly AT1-selective fluorescent ligand, based on losartan, that retains its antagonist activity with high affinity. Fluorescent derivatives of TRV027 become AT2-selective and lose their AT1 β-arrestin bias. These new ligands can be applied to trace AT1 or AT2 receptors in vitro and ex vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


