在铜基催化剂上通过卤素沉积将二氧化碳选择性氢化为甲醇
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
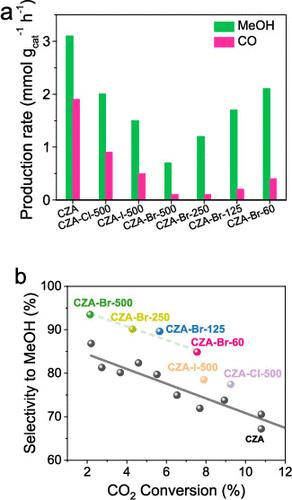
将二氧化碳加氢转化为甲醇是一条既能减少温室气体排放又能生产有价值的平台分子的可行途径。CuO-ZnO-Al2O3 (CZA) 催化剂在相对温和的条件下具有高活性,因此被用于以二氧化碳为原料生产甲醇。CO 的共生降低了 CO2 加氢过程中甲醇的选择性。在这项工作中,使用卤苯前驱体对 CZA 催化剂进行了卤素(Br、Cl 或 I)促进。与原始催化剂相比,溴的促进作用显著提高了甲醇选择性。在催化剂表面沉积不同数量的卤素时,都能观察到这种效果。结合表征技术和动力学分析,我们解释了卤素对催化性能的影响。在 CZA 催化剂中存在不同数量的卤素可通过两种方式提高甲醇选择性:抑制反向水气变换反应和阻碍甲醇分解为 CO。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective CO2 Hydrogenation to Methanol by Halogen Deposition over a Cu-Based Catalyst
The hydrogenation of carbon dioxide to methanol represents a promising pathway for both mitigating greenhouse gas emissions and producing valuable platform molecules. CuO-ZnO-Al2O3 (CZA) is the catalyst used for the methanol production from CO2 due to its high activity under relatively mild conditions. Coproduction of CO reduces the methanol selectivity in CO2 hydrogenation. In this work, the CZA catalyst has been promoted with halogens (Br, Cl, or I) using halobenzene precursors. The promotion with bromine significantly improves the methanol selectivity compared to the pristine catalyst. The effect was observed at different amounts of halogen deposited over the catalyst surface. A combination of characterization techniques and kinetic analysis enabled us to explain the effects of halogen on the catalytic performance. The presence of varying halogen amounts in the CZA catalyst enhances methanol selectivity in two ways: by suppressing the reverse water–gas shift reaction and by hindering methanol decomposition to CO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


