通过光氧化催化的双氰化过程实现异腈和(异)腈的 Csp3-Csp2 偶联
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在此,我们展示了异腈作为烷基自由基前体在光氧化催化转化过程中的应用能力,其中涉及选择性 C-N 裂解和 Csp3-Csp2 键的形成。该方案可通过脱氰过程,用容易获得的异腈制备功能化杂环戊烯。该反应适用于一级、二级和三级底物,包括氨基酸衍生物和类药物分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
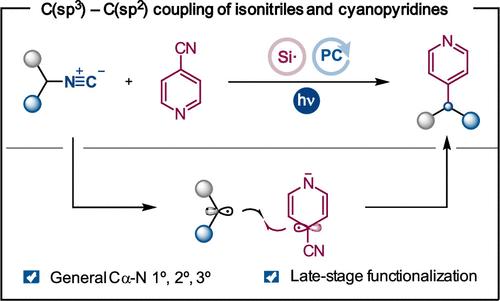
Csp3–Csp2 Coupling of Isonitriles and (Hetero)arenes through a Photoredox-Catalyzed Double Decyanation Process
Herein, we demonstrate the ability of isonitriles to be used as alkyl radical precursors in a photoredox-catalyzed transformation involving selective C–N cleavage and Csp3–Csp2 bond formation. This protocol allows for the preparation of functionalized heteroarenes from readily available isonitriles through a decyanation process. The reaction is general for primary, secondary, and tertiary substrates, including amino acid derivatives and druglike molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


