TYK2 在牛磺酸病小鼠模型中调控牛磺酸水平、磷酸化和聚集
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
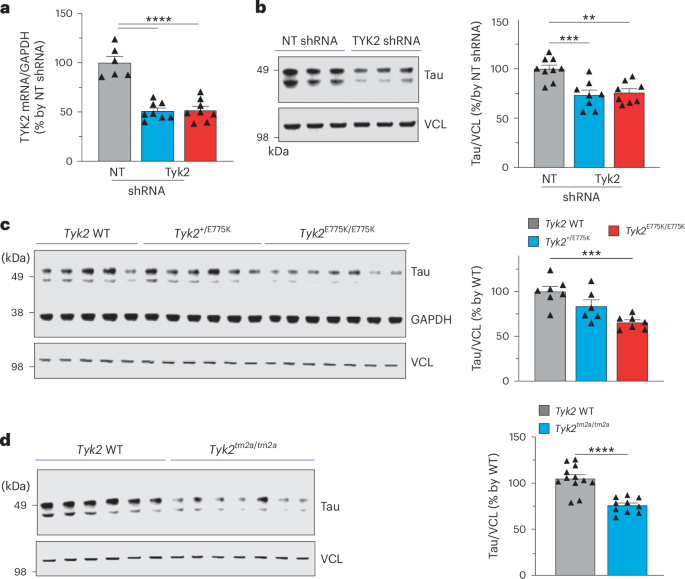
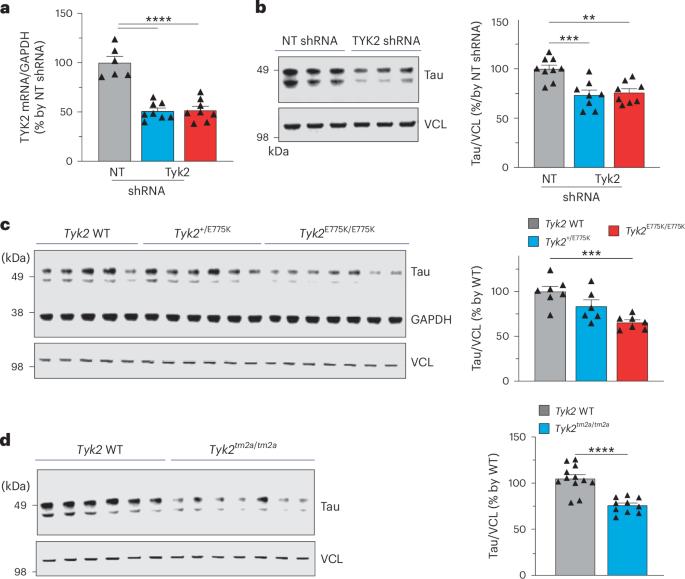
阿尔茨海默病是至少 26 种疾病之一,其特征是神经元、神经胶质细胞或两者中都有 tau 阳性聚集。然而,目前还不清楚是什么修饰导致可溶性 tau 转变为不溶性聚集体。我们之前进行了基因筛选,发现酪氨酸激酶2(TYK2)是tau水平的候选调节因子。在这里,我们验证了这一发现,并发现 TYK2 可使 tau 在酪氨酸 29 (Tyr29) 处磷酸化,导致其稳定并促进其在人体细胞中的聚集。我们发现,TYK2 介导的 Tyr29 磷酸化会干扰 tau 的自噬清除。我们还发现,在 P301S tau 转基因小鼠中,TYK2 介导的 Tyr29 磷酸化促进了病理性 tau 的积累。此外,Tyk2的敲除降低了总tau和致病性tau的水平,并挽救了tau病小鼠模型中的神经胶质病变。总之,这些数据表明,部分抑制TYK2可能是降低tau水平和毒性的一种策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
TYK2 regulates tau levels, phosphorylation and aggregation in a tauopathy mouse model
Alzheimer’s disease is one of at least 26 diseases characterized by tau-positive accumulation in neurons, glia or both. However, it is still unclear what modifications cause soluble tau to transform into insoluble aggregates. We previously performed genetic screens that identified tyrosine kinase 2 (TYK2) as a candidate regulator of tau levels. Here we verified this finding and found that TYK2 phosphorylates tau at tyrosine 29 (Tyr29) leading to its stabilization and promoting its aggregation in human cells. We discovered that TYK2-mediated Tyr29 phosphorylation interferes with autophagic clearance of tau. We also show that TYK2-mediated phosphorylation of Tyr29 facilitates pathological tau accumulation in P301S tau-transgenic mice. Furthermore, knockdown of Tyk2 reduced total tau and pathogenic tau levels and rescued gliosis in a tauopathy mouse model. Collectively, these data suggest that partial inhibition of TYK2 could thus be a strategy to reduce tau levels and toxicity. Tyrosine kinase 2 (TYK2) critically regulates tau levels and aggregation by phosphorylating tau’s tyrosine 29. Partial inhibition of TYK2 mitigates tau pathologies in cells and mice, highlighting TYK2 as a potential therapeutic target for Alzheimer’s disease and other tauopathies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


