为加速 COVID 计划建立和发展手性控制策略
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
nirmatrelvir 1(PAXLOVID 的活性药物)的开发采用了 "光速 "模式,以开发出一种治疗 SARS-CoV-2 的口服抗病毒药物 (COVID-19)。本文介绍了我们的手性控制策略,即在 17 个月的时间内,从首次人体研究到符合 ICH Q3A 标准的商业申报,提供高质量的药物物质。我们说明了我们的建模方法、有针对性的合成工作和分析方法开发,以测量最终药物物质中仅有的两种观察到的立体异构体,而不是潜在的 63 种。本文还概述了我们如何利用从 nirmatrelvir 手性控制中获得的知识,并将其应用于我们的第二代口服抑制剂 ibuzatrelvir 2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
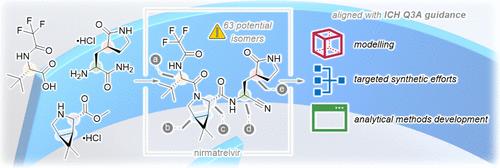
Building and Evolving a Chiral Control Strategy for Accelerated COVID Programs
The development of nirmatrelvir 1 (the active agent in PAXLOVID) was undertaken using a “lightspeed” paradigm to develop an oral antiviral treatment for SARS-CoV-2 (COVID-19). This paper describes our chiral control strategy to deliver high-quality drug substances from first in human studies to an ICH Q3A aligned commercial filing over a period of 17 months. We illustrate our approach to modeling, targeted synthetic efforts, and analytical method development to measure the only two observed stereoisomers instead of the potential 63 in the final drug substance. This paper also provides an overview of how we employed the knowledge gained on chiral control from nirmatrelvir and applied it to our second-generation oral inhibitor, ibuzatrelvir 2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


