α-氟化氧杂环的立体选择性开环反应:实践与理论研究
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
卤化物对氟化氧杂环丁烷(包括亚烷基氧杂环丁烷和螺环氧杂环丁烷)的开环反应具有高度的立体选择性,并由氟原子的存在所引导。该反应可立体选择性地制备四取代烯和含有全碳季中心的取代吡咯烷。为了阐明实验观察到的氧杂环丁烷衍生物打开过程中的区域选择性,我们进行了理论计算。为了比较形成不同非对映异构体的过渡态的能量,我们进行了过渡态计算。这些计算使用了多种 DFT 函数,并以 DLPNO-CCSD(T)/def2-TZVP 计算为基准。为了确认所发现的过渡态是否连接了反应物和生成物,还进行了本征反应配位(IRC)计算。然后,利用自然键轨道(NBO)分析法将 IRC 路径分解为静电、立体超共轭对反应障碍的贡献。破坏稳定的 Fδ----Br- 静电作用引导了反应路径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
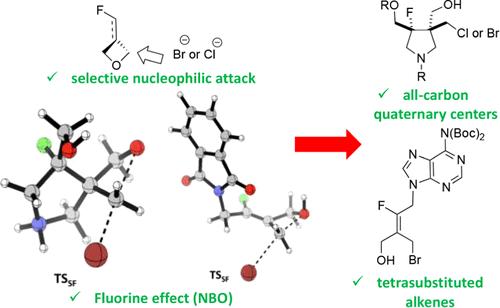
Stereoselective Ring-Opening Reaction of α-Fluorinated Oxetanes: A Practical and Theoretical Investigation
The ring-opening reaction of fluorinated oxetanes by halides, including alkylidene oxetanes and spirocyclic oxetanes, was highly stereoselective and directed by the presence of a fluorine atom. This reaction allowed a stereoselective preparation of tetrasubstituted alkenes and substituted pyrrolidines containing all-carbon quaternary centers. Theoretical calculations were performed to shed light on experimentally observed regioselectivity in the opening of oxetane derivatives. Transition state calculations were carried out to compare the energy of transition states responsible for forming different diastereoisomers. These calculations were performed using several DFT functionals and benchmarked to DLPNO–CCSD(T)/def2-TZVP calculations. Intrinsic reaction coordinated (IRC) calculations were run to confirm if the found transition states connect reactants and products. The IRC paths were then decomposed into the electrostatic, steric hyperconjugative contributions to reaction barriers by using the natural bond orbital (NBO) analysis. The destabilizing Fδ−···Br– electrostatic interaction directs the reaction pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


