星形胶质细胞转录组在阿尔茨海默病时空进展过程中的变化
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
星形胶质细胞对大脑稳态至关重要,但它们随着阿尔茨海默病(AD)神经病理学的时空进展而发生的变化仍未得到研究。在这里,我们对来自五个脑区的 628,943 个星形胶质细胞进行了单核 RNA 测序,这些细胞代表了从正常衰老到严重阿兹海默症病理过程中 32 个供体的阿兹海默症病理刻板进展。我们绘制出了几个独特的星形胶质细胞亚群,它们在AD易感神经网络(空间轴)或AD病理阶段(时间轴)对神经病理学表现出不同的反应。静态星形胶质细胞、中间星形胶质细胞和反应性星形胶质细胞的比例仅沿空间轴发生变化,而另外两个亚群则沿时间轴发生变化。其中一个富含营养因子的亚群在病理阶段下降,而另一个亚群在晚期增加,但在末期恢复到基线水平,这表明长期暴露于神经病理环境中会产生衰竭反应。我们的研究强调了 AD 中星形胶质细胞反应的复杂动态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
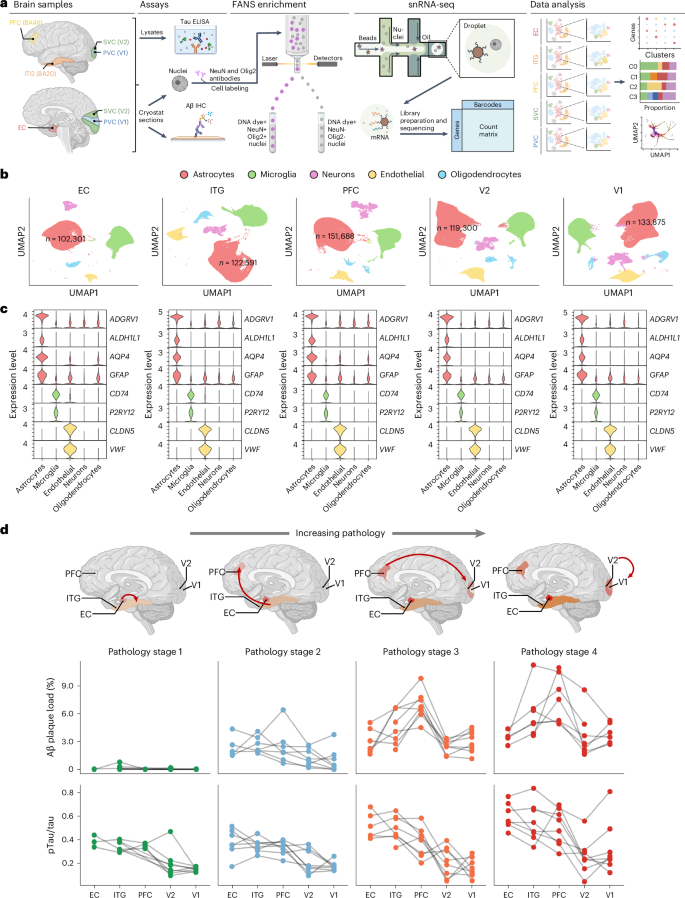
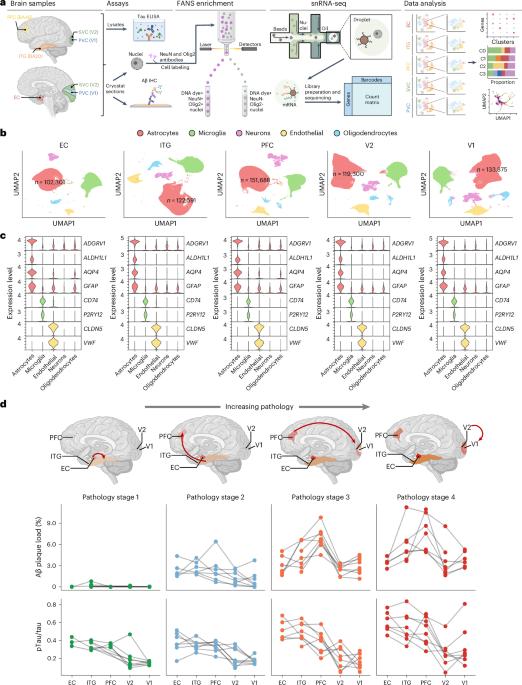
Astrocyte transcriptomic changes along the spatiotemporal progression of Alzheimer’s disease
Astrocytes are crucial to brain homeostasis, yet their changes along the spatiotemporal progression of Alzheimer’s disease (AD) neuropathology remain unexplored. Here we performed single-nucleus RNA sequencing of 628,943 astrocytes from five brain regions representing the stereotypical progression of AD pathology across 32 donors spanning the entire normal aging to severe AD continuum. We mapped out several unique astrocyte subclusters that exhibited varying responses to neuropathology across the AD-vulnerable neural network (spatial axis) or AD pathology stage (temporal axis). The proportion of homeostatic, intermediate and reactive astrocytes changed only along the spatial axis, whereas two other subclusters changed along the temporal axis. One of these, a trophic factor-rich subcluster, declined along pathology stages, whereas the other increased in the late stage but returned to baseline levels in the end stage, suggesting an exhausted response with chronic exposure to neuropathology. Our study underscores the complex dynamics of astrocytic responses in AD. Using single-nucleus RNA sequencing from 32 donors, researchers identified distinct astrocytic gene expression programs activated across brain regions and Alzheimer’s disease stages. They also found unique subclusters of astrocytes that appear to vary over time, highlighting the complexity of astrocytic responses in AD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


