通过脱插层促进 5-羟甲基糠醛在氢氧化镍上的电氧化作用
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电催化 5-羟甲基糠醛氧化反应(HMFOR)是绿色生产有价值含氧化学品的另一条途径。氢氧化镍由氢氧化物层和层间电荷平衡阴离子组成,是一种很有前景的 HMFOR 催化剂。在阐明氢氧化物层与 HMFOR 性能之间的相关性方面已经取得了进展,但插层阴离子对活性的影响仍不清楚。本文采用两种自支撑氢氧化镍催化剂(即原始 Ni(OH)2/CP-F 和脱插层 Ni(OH)2/CP-A)来揭示阴离子脱插与 HMFOR 活性之间的关系。物理特性分析表明,脱插现象可以改变 d 带中心并增加镍位点的电子密度。与原始 Ni(OH)2/CP-F 相比,这使得去交联的 Ni(OH)2/CP-A 具有更好的电化学性能(转化率 = 99.99 %;FDCA 产率 > 99 %;FE > 99 %)、更强的 HMF 吸附强度和更高的内在活性。这项研究不仅发现了一种性能优异的 HMFOR 电催化剂,还揭示了脱夹层对 Ni(OH)2 电化学氧化性能的重要影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
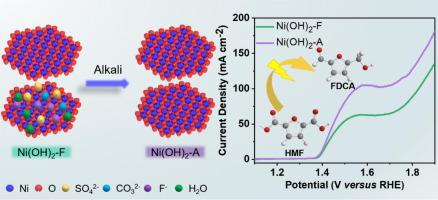
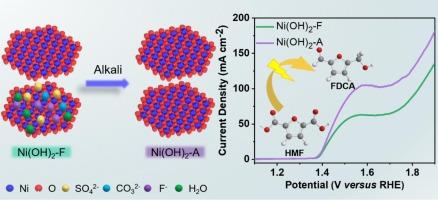
Facilitating the electrooxidation of 5-hydroxymethylfurfural on nickel hydroxide through deintercalation
The electrocatalytic of 5-hydroxymethylfurfural oxidation reaction (HMFOR) is an alternative route for the green production of valuable oxygenated chemicals. Nickel hydroxides, which consist of hydroxide layers and interlayer charge-balancing anions, are a type of promising catalysts for HMFOR. Progresses have been made on elucidating the correlation between the hydroxide layer and HMFOR performance, while the effect of intercalated anions on the activity remains unclear. Herein, two self-supported nickel hydroxide catalysts (i.e., pristine Ni(OH)2/CP-F and deintercalated Ni(OH)2/CP-A) are employed for revealing the relationship between anion-deintercalation and HMFOR activity. Physical characterizations demonstrate that the deintercalation phenomenon can alter the d-band center and increase the electron density of the Ni site. This endows the deintercalated Ni(OH)2/CP-A with improved electrochemical properties (conversion = 99.99 %; FDCA yield > 99 %; FE > 99 %), enhanced adsorption strength for HMF, and increased intrinsic activity, compared to the pristine Ni(OH)2/CP-F. This work not only reports an excellent HMFOR electrocatalyst, but also manifests the crucial effect of deintercalation on the electrochemical oxidation performance of Ni(OH)2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


