用柔性沸石咪唑啉框架-7 (ZIF-7) 吸附甲烷-氮混合物
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
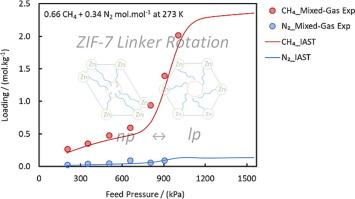
ZIF-7 是一种压力可调的柔性金属有机框架,相对于 N2 而言,它对 CH4 的吸入压力更低,因此极有可能抑制甲烷的逃逸性排放。在此,我们采用多种互补技术,对 ZIF-7 的改进型号在一定压力、温度和成分范围内从 N2 中分离 CH4 的情况进行了研究。在高达 16 兆帕的压力(283 至 323)K 条件下测量的纯气体吸附等温线表明,这种 ZIF-7 变体在大孔相中对 CH4 和 N2 的吸附能力比以前的 ZIF-7 样品高 50-60%。动态柱突破实验得出的二元混合物吸附等温线与利用理想吸附溶液理论根据纯气体等温线预测的结果一致。当达到混合物的孔隙相变压力时,甲烷的吸附量明显增加,而氮气的吸附量仍然很小。因此,ZIF-7 的平衡 CH4-over-N2 选择性始终大于 5.5,并可增至 9 左右,大大高于传统吸附剂。进一步的特性分析表明,ZIF-7 上的吸附速率最初会随着孔相转变的开始而降低,然后才会显著增加。沿吸附等温线在不同压力下采集的拉曼光谱显示了与结构变化相关的某些振动模式的变化。总之,这些观察结果表明,通常用来描述 ZIF-7 孔隙相变的概念简单的门控机制实际上取决于客体分子与吸附剂框架之间的相互作用,这种相互作用在材料进入大孔隙状态后继续起作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adsorption of Methane–Nitrogen mixtures with flexible Zeolitic Imidazolate Framework-7 (ZIF-7)
ZIF–7 is a pressure–regulated flexible metal–organic framework with a notably lower admission pressure for CH4 relative to N2, making it highly-prospective for curbing fugitive emissions of methane. Here, the separation of CH4 from N2 by an improved variant of ZIF–7 was studied over a range of pressures, temperatures, and compositions using multiple complementary techniques. Pure gas adsorption isotherms measured from (283 to 323) K at up to 16 MPa reveal this variant of ZIF-7 exhibits 50–60 % higher adsorption capacities for both CH4 and N2 in the large–pore phase than previous ZIF–7 samples. Binary mixture adsorption isotherms from dynamic column breakthrough experiments were consistent with those predicted from pure–gas isotherms using Ideal Adsorbed Solution Theory. Methane uptake increased significantly when the pore phase transition pressure for the mixture was reached, while the uptake of nitrogen remained small. Consequently, the equilibrium CH4-over–N2 selectivity of ZIF-7 is always larger than 5.5 and can increase to around 9, significantly above that of conventional adsorbents. Further characterisation showed the rate of the adsorption on ZIF–7 initially decreases as the pore phase transition begins before it increases significantly. Raman spectra acquired at various pressures along the adsorption isotherms revealed changes in certain vibrational modes associated with the structural change. Collectively, these observations indicate that the conceptually simple gating mechanism often used to describe the pore phase transitions in ZIF-7 in fact depends on interactions between the guest molecules and the adsorbent framework that continue to operate once the material is in the large–pore state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


