发现用于癌症治疗的新型大环非共价 CDK7 抑制剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
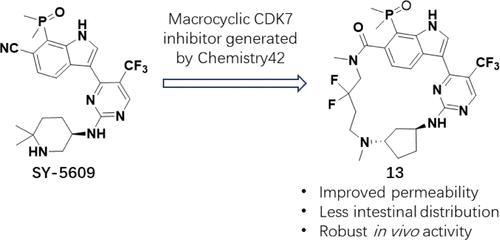
细胞周期蛋白依赖性激酶 7(CDK7)是细胞周期和转录的关键调控因子,因此是一种很有前景的癌症治疗靶点。尽管目前的 CDK7 抑制剂在选择性和类药物特性方面都有所改进,但由于严重的胃肠道和血液毒副作用,CDK7 抑制剂未能在临床开发中取得进展。为了缓解这些局限性,我们利用大环化平台开发出了新型、大环、非共价 CDK7 受体化合物 2 和 3,该平台从领先的临床资产 SY-5609 中优化了这些化合物。我们进行了广泛的结构-活性关系 (SAR) 研究,以提高其药效、增强口服生物利用度并减少肠道分布,最终得到了化合物 13。作为一种单一疗法,化合物 13 在异种移植模型中表现出了强效的体外活性、良好的 ADME 特性和强大的体内抗肿瘤活性。值得注意的是,碱度较低的化合物 13 改善了 Caco-2 的渗透性,降低了血液/血浆比率,减少了大鼠的肠道分布,从而减轻了胃肠道和血液毒性副作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of a Novel Macrocyclic Noncovalent CDK7 Inhibitor for Cancer Therapy
Cyclin-dependent kinase 7 (CDK7) is a key regulator of the cell cycle and transcription, making it a promising target for cancer therapy. Although current CDK7 inhibitors have improved in their selectivity and druglike properties, CDK7 inhibitors have failed to progress through clinical development due to severe gastrointestinal and hematotoxic side effects. To mitigate these limitations, we have developed novel, macrocyclic, noncovalent CDK7 hit compounds 2 and 3 using a macrocyclization platform that has optimized these compounds from SY-5609, a leading clinical asset. We conducted extensive structure–activity relationship (SAR) studies to improve their potency, enhance oral bioavailability, and reduce intestinal distribution, which resulted in compound 13. Compound 13 exhibits potent in vitro activity, good ADME properties, and robust in vivo antitumor activity in xenograft models as a monotherapy. Notably, compound 13 with lower basicity demonstrated improved Caco-2 permeability, reduced blood/plasma ratio, and reduced intestinal distribution in rats, thus mitigating gastrointestinal and hematotoxic side effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


