利用电化学和反应性二氧化碳捕获与矿化分离稀土元素和镍
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
目的是探究使用多功能溶剂同时捕获二氧化碳并将其转化为不溶性稀土元素(REE)碳酸盐,同时与镍形成可溶性络合物进行分离的化学机制。随后的镍电沉积可使装载二氧化碳的溶剂再生,以便再次使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
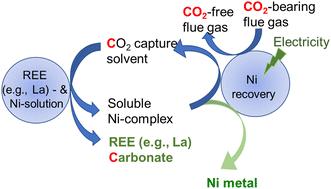
Separation of rare earth elements and nickel harnessing electrochemistry and reactive CO2 capture and mineralization†
The aim is to probe the chemical mechanisms underlying the use of multifunctional solvents to simultaneously capture and convert CO2 into insoluble rare earth element (REE)-carbonates, while forming soluble complexes with nickel for separation. Subsequent nickel electrodeposition regenerates the CO2-loaded solvent for reuse.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


