针对甲型流感病毒的 2-氨基吲哚衍生物的体外/体内合成和药效学评价
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
流感病毒是一种发病率和死亡率极高的呼吸道病原体,至今仍威胁着人类的健康。现有的抗流感药物存在各种局限性,如无法减轻身体损伤和副作用。目前仍急需开发一种新型抗病毒药物,在有效抑制病毒感染的同时避免对机体的伤害。研究人员通过 TMSOTf 催化 N-arylynamides 与亚磺酰亚胺的反应合成了一系列 2-氨基吲哚衍生物,并对其抗流感病毒活性进行了评估。实验结果表明,2-氨基吲哚 3h 在体外具有显著的抗病毒活性(EC50 = 8.37 ± 0.65 μM)和最低的细胞毒性(CC50 = 669.26 ± 11.42 μM)。2-Aminoindole 3h 可与 RNA 依赖性 RNA 聚合酶(RdRp)有效结合,从而抑制病毒复制,还可直接靶向宿主细胞,抑制病毒感染引起的细胞因子风暴和细胞凋亡,从而提高宿主细胞存活率。此外,2-氨基吲哚 3h 还能有效降低感染小鼠肺组织中的病毒载量和器官损伤,生物安全性令人满意。这些研究结果凸显了2-氨基吲哚作为抗流感病毒感染的治疗药物的潜力,同时也为该领域的进一步研究和开发奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
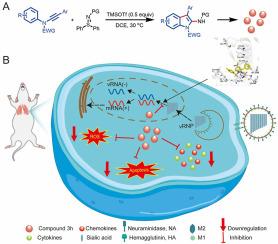
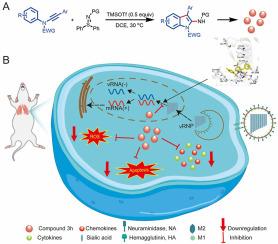
Synthesis and pharmacodynamic evaluation of 2-aminoindole derivatives against influenza A virus in vitro/vivo
Influenza virus is a kind of respiratory pathogen with high morbidity and mortality, which still threatens human health. Existing anti-influenza drugs have various limitations, such as the inability to alleviate body injury and side effects. There remains an urgent need to develop a novel antiviral drug to efficiently inhibit viral infection while avoiding body injury. A series of 2-aminoindole derivatives were synthesized via the TMSOTf-catalyzed reactions of N-arylynamides with sulfilimines and evaluated for their anti-influenza virus activity. The experimental results showed that 2-aminoindole 3h had significant antiviral activity (EC50 = 8.37 ± 0.65 μM) and the lowest cytotoxicity (CC50 = 669.26 ± 11.42 μM) in vitro. 2-Aminoindole 3h could inhibit viral replication by effectively binding to RNA-dependent RNA polymerase (RdRp), and could also directly target host cells to inhibit cytokine storms and apoptosis induced by viral infection, thereby improving host cell survival rate. In addition, viral load and organ injury in the lung tissue of infected mice were effectively reduced by 2-aminoindole 3h with satisfactory biosafety. These findings highlight the potential of a valuable therapeutic option against influenza infection while also laying the foundation for further research and development in this area.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


