作为选择性醛糖还原酶抑制剂的新型 N-苄基吲哚类依帕司他类似物的合理设计与合成:作为线粒体解偶联剂的新型降糖药(AK-4)的意外发现
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
糖尿病是与高血糖相关的最常见代谢性疾病之一。虽然抗糖尿病药物能降低高血糖,但糖尿病患者的血糖水平会出现异常波动,导致长期并发症的发生。醛糖还原酶抑制剂被认为是调节糖尿病特异性并发症发生的一种有前途的策略。迄今为止,依帕司他是亚洲国家唯一获批的药物。在本文中,我们的研究立足于发现新型依帕司他类似物,以预防慢性并发症并使高血糖正常化。在本文中,我们介绍了四种新型 4-噻唑烷酮乙酸衍生物(AK-1-4)的合理设计与合成,并评估了它们对大鼠镜片中醛糖还原酶的疗效及其对大鼠肾脏中同源酶的特异性。AK-1-4 还针对人重组蛋白酪氨酸磷酸酶 1B 进行了测试,该酶是胰岛素增敏作用的关键靶点,AK-1-4 还针对与之密切相关的 T 细胞衍生酶进行了测试。对接分析表明了与所研究靶点的可能结合模式。AK-4 具有良好的抑制作用,这激发了我们探索其对胰岛素受体信号通路的影响及其在体内外条件下刺激葡萄糖摄取的能力的兴趣。我们进一步研究了 AK-4 作为解偶联剂靶向线粒体和损害线粒体膜电位的能力。在此,我们首次报道了一种新型降糖药物(AK-4),它通过控制线粒体解偶联活性,既能缓解慢性糖尿病并发症,又不会产生脱靶不良反应,同时还具有降血糖功效。硅学药代动力学和毒性研究显示,AK-4 具有最佳口服特性,且无潜在副作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
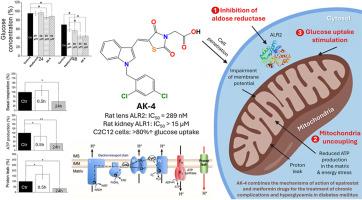
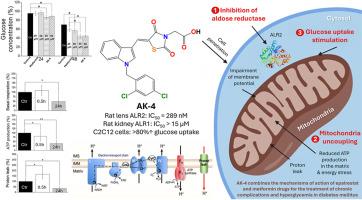
Rational design and synthesis of novel N-benzylindole-based epalrestat analogs as selective aldose reductase inhibitors: An unexpected discovery of a new glucose-lowering agent (AK-4) acting as a mitochondrial uncoupler
Diabetes mellitus is one of the most frequent metabolic diseases associated with hyperglycemia. Although antidiabetic drugs reduce hyperglycemia, diabetic patients suffer from abnormal fluctuations in blood glucose levels leading to the onset of long-term complications. Aldose reductase inhibitors are considered a promising strategy for regulating the occurrence of diabetic-specific comorbidities. So far, epalrestat is the only drug being approved in Asian countries. In this paper, we ground our research in discovering novel epalrestat analogs that prevent chronic complications and normalize hyperglycemia. Herein, we describe the rational design and synthesis of four novel 4-thiazolidinone acetic acid derivatives (AK-1-4) being evaluated for their efficacy against aldose reductase from rat lenses and their specificity over the homologous enzyme from rat kidneys. AK-1-4 were also tested against human recombinant protein tyrosine phosphatase 1B as a key target in insulin sensitization and towards the closely related T-cell-derived enzyme. Docking analyses suggested possible binding modes on examined targets. The promising inhibitory profile of AK-4 sparked our interest in exploring its effect on the insulin-receptor signaling pathway and its ability to stimulate glucose uptake under ex vivo conditions. We further investigated the ability of AK-4 to target mitochondria acting as an uncoupling agent and impairing mitochondrial membrane potential. Herein, we report for the first time a new glucose-lowering agent (AK-4) that can combine alleviation for chronic diabetic complications without off-target adverse effects and antihyperglycemic efficacy through controlled mitochondrial uncoupling activity. Pharmacokinetic and toxicity studies in silico revealed optimal properties of AK-4 for oral administration without potential side effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


