一系列强效、选择性和药物样 G 蛋白偶联受体激酶 5 抑制剂的设计、合成和 X 射线结构研究
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
G蛋白偶联受体激酶5(GRK5)已成为治疗心力衰竭和癌症的潜在药物开发靶点。GRK6的同源物是多发性骨髓瘤的治疗靶点。我们合理地设计了一系列高选择性、强效、非共价和类药物 GRK5 抑制剂。几种抑制剂对 GRK5 的抑制作用低至纳摩尔,对 GRK2 有高选择性,令人惊讶的是,有些抑制剂对 GRK6 也有选择性。我们测定了几种抑制剂与 GRK5 复合物的高分辨率 X 射线晶体结构,从而从分子角度揭示了导致 GRK5 选择性和效力的配体-结合位点相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
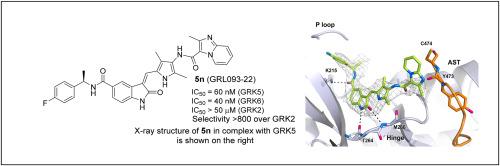
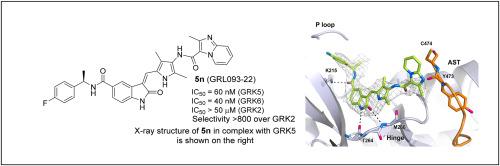
Design, synthesis, and X-ray structural studies of a series of highly potent, selective, and drug-like G protein-coupled receptor kinase 5 inhibitors
G protein-coupled receptor kinase 5 (GRK5) has emerged as a potential drug development target against heart failure and cancer. A close homolog, GRK6 represents a therapeutic target for multiple myeloma. We have rationally designed a series of highly selective, potent, noncovalent, and drug-like GRK5 inhibitors. Several inhibitors exhibited low nanomolar GRK5 inhibition and high selectivity over GRK2, and, surprisingly, some were selective for GRK6. We determined high-resolution X-ray crystal structures of several inhibitors in complex with GRK5, which provide molecular insights into the ligand-binding site interactions responsible for GRK5 selectivity and potency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


