将二芳基胺衍生物合成为治疗阿尔茨海默病的 Tau-PET 放射性示踪剂并进行临床前评估
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
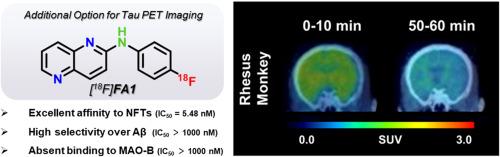
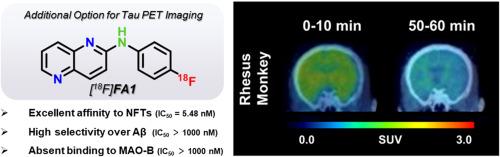
大脑中存在聚集的 Tau 是 Tau 病,尤其是阿尔茨海默病(AD)的主要病理特征。因此,开发能特异、灵敏地与 Tau 聚集体结合的配体对于诊断和监测治疗干预措施至关重要。在本研究中,我们进一步研究了二芳基胺骨架的结构优化,它表现出了良好的结合特性和生物特性。我们还探索了氮原子的数量和位置、杂原子和芳香分子的类型以及放射性位置对 Tau 亲和力的影响。通过基于 125I 标记的二芳基胺衍生物的结构-活性关系(SAR)分析,[125I]A6 因其理想的结合特性和穿透大脑的能力而被确定为先导化合物,使其适合转化为 18F 标记的 PET 示踪剂。经啮齿动物和非人灵长类动物的动态 PET 研究证实,[18F]FA1 满足了 Tau 放射性示踪剂的关键要求,对 Tau 具有高度的特异性和选择性,对 Aβ 斑块和单胺氧化酶 B (MAO-B) 具有良好的脱靶特性,并且具有良好的体内脑动力学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and preclinical evaluation of diarylamine derivative as Tau-PET radiotracer for Alzheimer's Disease
The presence of aggregated Tau in the brain is a dominant pathological hallmark of Tauopathies, particularly in Alzheimer's disease (AD). Therefore, developing ligands that can specifically and sensitively bind to Tau aggregates is essential for diagnosing and monitoring therapeutic interventions. In this study, we further investigated the structural optimization of the diarylamine skeleton, which exhibited promising binding characteristics and biological properties. We supplementarily explored the effects of the number and position of nitrogen atoms, types of heteroatoms and aromatic moieties, and radioactive positions on affinity for Tau. Through a structure-activity relationship (SAR) analysis based on 125I-labeled diarylamine derivatives, [125I]A6 was identified as a lead compound due to its desirable binding properties and ability to penetrate the brain, making it suitable for conversion into a18F-labeled PET tracer. Satisfactorily, [18F]FA1 fulfilled critical requirements as a Tau radiotracer, demonstrating high specificity and selectivity for Tau, a clean off-target profile against Aβ plaques and monoamine oxidase B (MAO-B), and favorable in vivo brain kinetics, as confirmed by dynamic PET studies in rodents and non-human primates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


