3 芳基吲哚与融合吲哚体系的脱氢光环化反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过无催化剂光引发的脱氢环化转化,以 3-苯乙烯基吲哚为原料合成了 5-芳基或 5,6- 二芳基-11H-苯并[a]咔唑,在温和的条件下以优异的收率提供了一系列结构多样的产物。该方案也适用于呋喃和噻吩样品。这种马洛里型反应在氩气环境中进行,无需外加氧化剂。最后,还进行了详细的机理研究,动力学同位素效应实验表明,脱氢环化反应涉及光诱导的 6π 电环闭环和氢进化级联过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
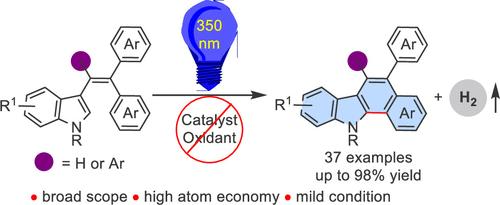
Dehydrogenative Photocyclization of 3-Styryl Indoles to Fused Indole Systems
The synthesis of 5-aryl- or 5,6-diaryl-11H-benzo[a]carbazoles has been achieved from 3-styryl indoles through catalyst-free photoinitiated dehydrogenative cyclization transformation, providing a range of structurally diverse products in excellent yields under mild conditions. The protocol is also applicable to furan and thiophene samples. This Mallory-type reaction takes place in an argon atmosphere without external oxidants. Finally, detailed mechanistic studies were performed, and kinetic isotope effect experiments indicate that dehydrogenative annulation reaction involves photoinduced 6π-electrocyclic ring-closing and hydrogen evolution cascade processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


