循环肿瘤 DNA 状态和动态变化可预测切除肝外胆管癌患者的复发情况
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
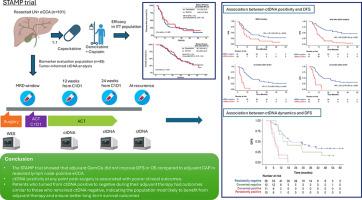
背景& 目的手术是可切除肝外胆管癌(eCCA)唯一的根治性治疗方案,但复发很常见,预后较差。改善辅助化疗(ACT)决策的临床需求尚未得到满足。在此,我们评估了监测纵向循环肿瘤DNA(ctDNA)对STAMP试验患者的最小残留病(MRD)的作用,该试验比较了卡培他滨(CAP)与吉西他滨加顺铂(GemCis)的辅助疗效。疗效结果分析的随访时间比之前的报告延长了19个月。在89例患者的生物标志物队列中,前瞻性地收集了手术后辅助化疗(ACT)前的纵向血浆样本(n=254),以及自第1周期第1天(C1D1)起12周和24周的ACT时的血浆样本。结果在延长的随访分析中,CAP组和GemCis组的中位无病生存期(DFS)和总生存期(OS)没有显著差异。ctDNA阳性与ACT前(HR,1.8;p=0.029)、C1D1起12周的ACT时(HR,7.72;p<0.001)、C1D1起24周的ACT时(HR,5.24;p<0.001)和手术后任何时间(HR,3.81;p<0.001)的DFS显著降低有关。对治疗前到治疗中的ctDNA动态分析显示,与ctDNA持续阳性(HR,6.7;p<0.001)或ctDNA转阳(HR,5.8;p<0.001)的患者相比,ctDNA连续阴性患者的DFS明显更长。影响和意义本研究结果强调了ctDNA作为预后生物标志物和监测工具在肝外胆管癌(eCCA)患者中的重要作用。通过证明ctDNA在预测疾病复发方面优于CA 19-9和CEA等传统生物标志物,该研究强调了ctDNA在指导辅助化疗决策方面的潜力。这些结果可能对完善术后治疗策略和改善患者预后至关重要。ctDNA监测的实际应用可能会带来更个性化的治疗方法,从而能够根据最小残留病(MRD)状态进行及时干预。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Circulating tumor DNA status and dynamics predict recurrence in patients with resected extrahepatic cholangiocarcinoma
Background & Aims
Surgery is the only curative therapeutic option for resectable extrahepatic cholangiocarcinoma, but recurrence is common, and prognosis is poor. There is an unmet clinical need for improved decision-making regarding adjuvant chemotherapy (ACT). Herein, we evaluated the usefulness of monitoring longitudinal circulating tumor DNA (ctDNA) for molecular residual disease (MRD) in patients from the STAMP trial, which compared the efficacy of adjuvant capecitabine (CAP) vs. gemcitabine plus cisplatin (GemCis).
Methods
Between July 2017 and November 2020, 101 patients were randomized 1:1 to receive GemCis (n = 50) or CAP (n = 51). Efficacy outcomes were analyzed with an extended follow-up of 19 months from the previous report. From a biomarker cohort of 89 patients, longitudinal plasma samples (n = 254) were prospectively collected post-surgery before ACT, and on-ACT at 12 and 24 weeks from cycle 1 day 1 (C1D1). ctDNA was evaluated using a personalized, tumor-informed, 16-plex PCR next-generation sequencing assay and was correlated with clinical outcomes.
Results
In the extended follow-up analysis, median disease-free survival (DFS) and overall survival did not significantly differ between the CAP and GemCis groups. Significantly inferior DFS was associated with ctDNA positivity before ACT (hazard ratio [HR] 1.8; p = 0.029), on-ACT at 12 weeks from C1D1 (HR 7.72; p <0.001), on-ACT at 24 weeks from C1D1 (HR 5.24; p <0.001), and anytime post-surgery (HR 3.81; p <0.001). Analysis of pre-treatment to on-treatment ctDNA dynamics revealed that serially ctDNA-negative patients exhibited a significantly longer DFS compared to those with sustained ctDNA positivity (HR 6.7; p <0.001) or those who turned ctDNA positive (HR 5.8; p <0.001).
Conclusion
In patients with resected extrahepatic cholangiocarcinoma, ctDNA status and dynamics predicted recurrence during adjuvant therapy, and may help optimize clinical decision-making.
Impact and implications
The findings from this study highlight the critical role of ctDNA as a prognostic biomarker and monitoring tool for patients with resected extrahepatic cholangiocarcinoma. By demonstrating the superiority of ctDNA to predict disease recurrence compared to conventional biomarkers such as cancer antigen 19-9 and carcinoembryonic antigen, this study underscores its potential in guiding decision-making during adjuvant chemotherapy. These results may be crucial to refine post-surgical treatment strategies and improve patient outcomes. The practical application of ctDNA monitoring could lead to more personalized treatment approaches, enabling timely interventions based on molecular residual disease status.
Clinical Trial Registration number
NCT03079427.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


