用于氢气进化的固溶中熵合金纳米粒子的室温电化学冲击合成技术
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
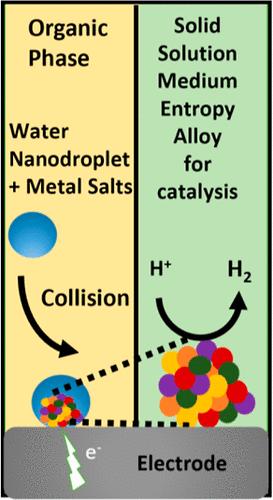
千百年来,人类发现了合金材料的巨大益处。在过去的 100 年中,纳米科学的复兴巩固了纳米粒子在从能量存储和转换到细胞生物学等各个领域的重要性。虽然已有许多合成纳米粒子和合金纳米粒子的策略,但要在前所未有的条件下制造纳米粒子,还需要新的方法。在这里,我们展示了一种在室温下电沉积固溶体合金纳米粒子的简单技术。当铂、金和钯的金属盐被限制在悬浮于油中的纳米液滴中,然后纳米液滴与足够负偏压的电极碰撞,使金属盐在传质限制下还原,就形成了固溶体合金纳米粒子。高角度环形暗场扫描透射电子显微镜和单原子能量色散 X 射线光谱证实了纳米颗粒的固溶微观结构。这些结果还证实了纳米液滴能够将合金微观结构从非晶态调整为固溶态。我们通过添加银盐进一步扩展了我们的技术,从而合成了多晶中熵合金。最后,我们通过强调中熵合金对氢进化反应的电催化活性,展示了它在可再生能源和清洁能源设备方面的应用。我们的方法具有无与伦比的简便性,可应用于各个研究领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Room Temperature Electrochemical-Shock Synthesis of Solid-Solution Medium-Entropy Alloy Nanoparticles for Hydrogen Evolution
For millennia, humankind has discovered great benefits in alloying materials. Over the past 100 years, a renaissance in nanoscience has cemented the importance of nanoparticles in a variety of fields ranging from energy storage and conversion to cell biology. While many synthetic strategies exist for nanoparticle and alloy nanoparticle formation, new methods are necessary to create nanoparticles under unprecedented conditions. Here, we demonstrate a simple technique to electrodeposit solid-solution alloy nanoparticles at room temperature. When metal salts of platinum, gold, and palladium are confined to nanodroplets suspended in oil, and then a nanodroplet collides with a sufficiently negative-biased electrode to reduce the metal salts at the mass-transfer limitation, solid-solution alloy nanoparticles form. High-angle annular dark-field scanning transmission electron microscopy and single atom energy dispersive X-ray spectroscopy confirm the solid-solution microstructure of the nanoparticles. The results also confirm the nanodroplet’s ability to tune alloy microstructures from amorphous to solid-solution. We further extend our technique by adding salts of silver, which lead to the synthesis of polycrystalline medium-entropy alloys. Finally, we go on to show the application of our midentropy alloys toward renewable and clean energy devices by highlighting their electrocatalytic activity toward hydrogen evolution reaction. Our method is unrivaled in its simplicity and will find applications across various fields of study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


