用于胶质母细胞瘤靶向催化免疫疗法的仿生双驱动异质结纳米马达
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胶质母细胞瘤(GBM)中血脑屏障(BBB)的存在和免疫抑制微环境的特点为GBM的靶向治疗带来了重大挑战。为解决这一问题,我们提出了一种用于 GBM 靶向治疗的仿生混合细胞膜修饰双驱动异质结纳米马达(HM@MnO2-AuNR-SiO2)。这些纳米马达通过模仿 GBM 和巨噬细胞膜的表面特征,可绕过 BBB 并靶向胶质瘤区域。更重要的是,MnO2-AuNR-SiO2 异质结结构可通过近红外-II(NIR-II)光和氧气泡实现双驱动推进,从而有效治疗深部肿瘤部位。同时,等离子体 AuNR-MnO2 异质结结构可促进电子-空穴对的分离,并产生活性氧(ROS),在近红外-II 激光照射下诱导免疫性肿瘤细胞死亡。此外,肿瘤微环境中的二氧化锰会发生反应,释放出 Mn2+ 离子,激活 cGAS-STING 通路,增强抗肿瘤免疫力。体外和体内实验证明,这些双驱动生物仿生纳米马达可实现主动靶向和肿瘤深层浸润,促进 M1 巨噬细胞极化、树突状细胞成熟和效应 T 细胞活化,从而通过产生 ROS 和激活 STING 通路增强 GBM 催化和免疫治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
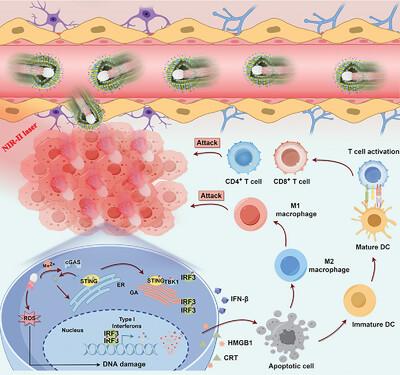
Biomimetic Dual‐Driven Heterojunction Nanomotors for Targeted Catalytic Immunotherapy of Glioblastoma
The existence of the blood–brain barrier (BBB) and the characteristics of the immunosuppressive microenvironment in glioblastoma (GBM) present significant challenges for targeted GBM therapy. To address this, a biomimetic hybrid cell membrane‐modified dual‐driven heterojunction nanomotor (HM@MnO2 ‐AuNR‐SiO2 ) is proposed for targeted GBM treatment. These nanomotors are designed to bypass the BBB and target glioma regions by mimicking the surface characteristics of GBM and macrophage membranes. More importantly, the MnO2 ‐AuNR‐SiO2 heterojunction structure enables dual‐driven propulsion through near‐infrared‐II (NIR‐II) light and oxygen bubbles, allowing effective treatment at deep tumor sites. Meanwhile, the plasmonic AuNR‐MnO2 heterostructure facilitates the separation of electron–hole pairs and generates reactive oxygen species (ROS), inducing immunogenic tumor cell death under NIR‐II laser irradiation. Furthermore, MnO2 in the tumor microenvironment reacts to release Mn2+ ions, activating the cGAS‐STING pathway and enhancing antitumor immunity. In vitro and in vivo experiments demonstrate that these dual‐driven biomimetic nanomotors achieve active targeting and deep tumor infiltration, promoting M1 macrophage polarization, dendritic cell maturation, and effector T‐cell activation, thereby enhancing GBM catalysis and immunotherapy through ROS production and STING pathway activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


