定制二维导电共邻苯二酚金属有机框架的电催化氧进化反应性能
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
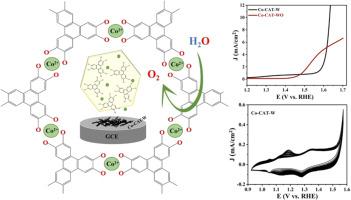
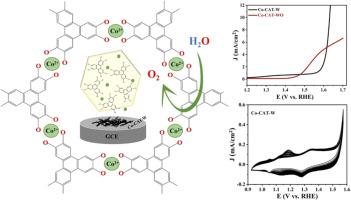
在此,我们报告了在无 N-甲基-2-吡咯烷酮(NMP)极性溶剂(Co-CAT-WO)和有 N-甲基-2-吡咯烷酮(NMP)极性溶剂(Co-CAT-W)的条件下微波水热合成高多孔性和导电性二维 Co-catecholate MOFs(Co-CATs)的过程,以及将这些 Co-CATs 用作氧进化反应(OER)电催化剂的研究。比较了 Co-CAT-WO 和 Co-CAT-W 的氧进化反应性能。合成的 Co-CAT 呈二维层状六边形结构。Co-CAT-WO 和 Co-CAT-W 的导电率分别为 6.9 和 5.8 S/m。良好的导电性和多孔结构可以启动电荷传输,从而在 4e 转移过程中实现更好的 OER 性能。在 1M KOH 中进行 13 小时的耐久性和时变实验后,制备的 Co-CAT-WO 和 Co-CAT-W 在 10 mA/cm2 下的过电位分别为 455 mV 和 424 mV。从电化学研究中可以明显看出,Co-CAT-W 具有更好的 OER 性能,过电位低、电流密度高,并且在长时间循环中具有出色的稳定性。此外,与 Co-CAT-WO 相比,Co-CAT-W 的 HOMO-LUMO 间隙更低,这也证明它具有更好的电催化活性。OER后的研究结果表明,Co-CAT-W电催化剂起着 "前催化剂 "而非原催化剂的作用,它在OER研究后会发生电化学转化,变成金属氢氧化物和金属氧氢氧化物,从而促进了OER动力学的发展。这项工作的发现为开发二维导电金属-儿茶酚 MOF 催化剂的合成策略提供了有价值的见解,这些催化剂可用于高效和可持续的 OER 过程,而这在水分离以实现可持续能源生产方面至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tailoring of electrocatalytic oxygen evolution reaction performance of 2D conductive Co-catecholate metal-organic frameworks
Herein, we report the microwave hydrothermal synthesis of highly porous and conductive 2D Co-catecholate MOFs (Co-CATs) in the absence (Co-CAT-WO) and presence (Co-CAT-W) of N-methyl-2-pyrrolidone (NMP) polar solvent, and the study of these Co-CATs as electrocatalysts for oxygen evolution reaction (OER). The OER performance of Co-CAT-WO and Co-CAT-W is compared. The as-synthesized Co-CATs exhibit a 2D layered hexagonal structure. The electrical conductivity of Co-CAT-WO and Co-CAT-W is 6.9 and 5.8 S/m, respectively. The good conductivity and porous structure can initiate charge transport to achieve better OER performance under the 4e--transfer process. The as-prepared Co-CAT-WO and Co-CAT-W, respectively, show an overpotential of 455 and 424 mV at 10 mA/cm2 after performing the durability and chronoamperometry experiments for 13 h in 1 M KOH. From the electrochemical studies, it is apparent that the Co-CAT-W exhibits better OER performance with low overpotential, high current density, and excellent stability over extended cycling. Moreover, the lower HOMO-LUMO gap for Co-CAT-W than that of the Co-CAT-WO endorses its better electrocatalytic activity. The post-OER results show that the Co-CAT-W electrocatalyst acts as a "precatalyst" rather than the original catalyst, and it undergoes electrochemical transformation to metal hydroxide and metal oxyhydroxide after the OER studies, promoting the OER kinetics. The findings of this work offer valuable insights into the synthetic strategies for developing 2D conducting metal-catecholate MOF catalysts for efficient and sustainable OER processes, which is crucial in water splitting for sustainable energy production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


