耦合(CuO-Co3O4)与混合(CuCo2O4)金属氧化物电催化效率的比较研究:通过肼氧化和灵敏度测定进行探究
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
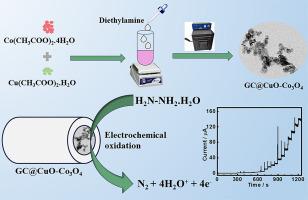
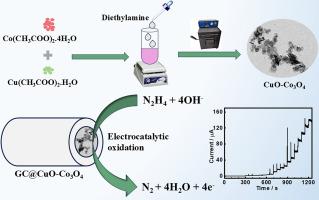
本研究报告采用简单的水热法和煅烧法合成了铜基和钴基耦合金属氧化物和混合金属氧化物(分别为 CuO-Co3O4 和 CuCo2O4)。通过粉末 X 射线衍射 (XRD)、高分辨率透射电子显微镜 (HR-TEM)、场发射扫描电子显微镜 (FE-SEM)、能量色散 X 射线和 X 射线光电子能谱对 CuO-Co3O4、CuCo2O4 和对照样品进行了表征。对 CuO-Co3O4 的 TEM 和 FE-SEM 分析显示出两种不同的形态:杆状和球状颗粒(分别为 CuO 和 Co3O4)。此外,CuO-Co3O4 被有效地用作选择性氧化肼(Hyz)的电催化剂。与 CuO、Co3O4、CuCo2O4 以及 CuO 和 Co3O4 的物理混合物(CuO/Co3O4)相比,CuO-Co3O4 显示出较高的氧化还原反应。这种性能的提高归因于金属离子之间的协同作用,这种作用是由它们之间的接近和表面活性位点的暴露增加引起的。在测定 Hyz 时,CuO-Co3O4 具有宽线性范围(1-3500 µM)、低检测限(0.29 µM)和高灵敏度(0.5756 µA µM-1 cm-2)。利用计时器篡改法获得了动力学参数,例如 Hyz 氧化的扩散系数和催化速率常数。此外,CuO-Co3O4 被有效地用于分析实际样品中的 Hyz,且回收率可接受。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A comparative study on the electrocatalytic efficiency of coupled (CuO-Co3O4) vs. mixed (CuCo2O4) metal oxides: Probed by hydrazine oxidation and sensitive determination
This work reports the synthesis of copper- and cobalt-based coupled and mixed metal oxides (CuO-Co3O4 and CuCo2O4, respectively) utilizing a simple hydrothermal and calcination approach. CuO-Co3O4, CuCo2O4, and the control samples were characterized by powder X-ray diffraction (XRD), high-resolution transmission electron microscopy (HR-TEM), field emission scanning electron microscopy (FE-SEM), energy dispersive X-ray, and X-ray photoelectron spectroscopy. TEM and FE-SEM analyses of CuO-Co3O4 reveal the presence of two distinct morphologies: rod- and sphere-shaped particles (CuO and Co3O4, respectively). Further, CuO-Co3O4 was efficiently utilized as an electrocatalyst for the selective oxidation of hydrazine (Hyz). CuO-Co3O4 shows a high redox response compared to CuO, Co3O4, CuCo2O4, and the physical mixture of CuO and Co3O4 (CuO/Co3O4). This enhanced performance is attributed to the synergistic interaction between the metal ions caused by their close proximity and the increased exposure of surface active sites. CuO-Co3O4 shows a broad linear range (1–3500 µM), a low detection limit (0.29 µM), and high sensitivity (0.5756 µA µM-1 cm-2) for the Hyz determination. Kinetic parameters, for instance the diffusion coefficient and catalytic rate constant for Hyz oxidation were obtained using chronoamperometry. Additionally, CuO-Co3O4 was effectively utilized to analyze Hyz in real samples with acceptable recovery rates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


