对非那西酸伏安法的批判性分析:双氯芬酸和甲非那酸电活性膜形成的证据
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
双氯芬酸和甲灭酸在丝网印刷碳电极上的伏安行为非常相似,这两种化合物的分子结构与二苯胺相似。在每种情况下,经过反复扫描后,由于其中一种分子的聚合作用,都会沉积出一层电活性薄膜。由此形成的薄膜附着在电极上,双氯芬酸和甲芬那酸在水溶液中都很稳定。这种薄膜的形成在碳电极上是独一无二的,因为在铂或金电极上不会形成薄膜层。封闭层的形式电位随 pH 值变化,斜率约为 -59mV/pH。除非在两次运行之间对电极进行清洁,否则这些层将妨碍在定量分析中多次使用该电极。本文提出了一个基于二苯胺骨架形成聚合物的模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
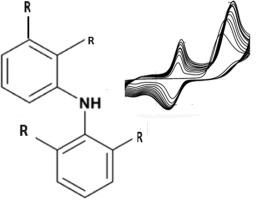
A Critical Analysis of the Voltammetry of Fenamic acids: Evidence of diclofenac and mefenamic acid electroactive film formation
The voltammetric behaviour of diclofenac and mefenamic acid at a screen printed carbon electrode is very similar and both compounds share a similar molecular structure to that of diphenylamine. In each case on repeated scanning, an electroactive film is deposited due to the polymerisation of either of the molecules. The resultant film adheres to the electrode and is stable in aqueous solution for both diclofenac and mefenamic acid. This film formation is unique to a carbon electrode as no layers were found to form on Pt or Au. The formal potential of the confined layer shifts with pH, with a slope of about -59mV/pH unit. Such layers will prevent the multiple use of the electrode in quantitative analysis unless it is cleaned between runs. A model based on the polymer formation from a diphenylamine backbone is proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


