基于 IrO2 催化剂的铱位点活化,实现高度稳定的 PEM 水电解槽
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在质子交换膜(PEM)水电解槽中,开发具有高活性和稳定性的阳极电催化剂仍然是一项巨大的挑战。在此,我们通过熔盐密封法掺杂锰以激活 IrO2 中的 Ir 位点,优化后的 Mn0.1Ir0.9O2 表现出优异的 OER 性能。掺入的锰优化了IrO2的电子结构和主要晶面,这一点在我们的互补表征中得到了证明。令人印象深刻的是,Mn0.1Ir0.9O2 在 PEM 水电解槽中表现出了卓越的性能,只需要 1.79 V 就能达到 1 A cm-2,并能在 1200 小时内保持长期稳定性(衰减率仅为 17.5 μV h-1,而原始样品的衰减率为 1.27 mV/h)。DFT 计算表明,锰掺杂可激活 Ir 位点,从而降低促进 OER 的速率决定步骤的能垒。这项工作为在实际工作条件下为 PEM 水电解槽设计高耐受性催化剂提供了一种策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
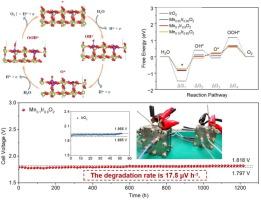
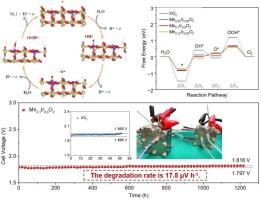
Activation of iridium site based on IrO2 catalysts towards highly stable PEM water electrolyzer
The exploitation of anode electrocatalysts with high activity and stability remains an enormous challenge in proton exchange membrane (PEM) water electrolyzer. Here we doped Mn to activate Ir site in IrO2 through the molten salt sealing method, and the optimized Mn0.1Ir0.9O2 exhibited superior OER performance. Mn doping optimized the electronic structure and dominant crystal planes of IrO2, as evidenced by our complementary characterizations. Impressively, the Mn0.1Ir0.9O2 exhibits superior performance in the PEM water electrolyzer, only requiring 1.79 V to attain 1 A cm−2 and offering long-term stability over 1200 h (attenuated only 17.5 μV h−1 compared to 1.27 mV/h for the original sample). DFT calculation shows Mn doping could activate Ir site, thus reducing the energy barrier of the rate-determining step for promoting OER. This work provides a strategy to design high-tolerance catalysts for the PEM water electrolyzer in actual working conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


