质子氢键和氢ridic 氢键虽来源不同,但光谱表现相似
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
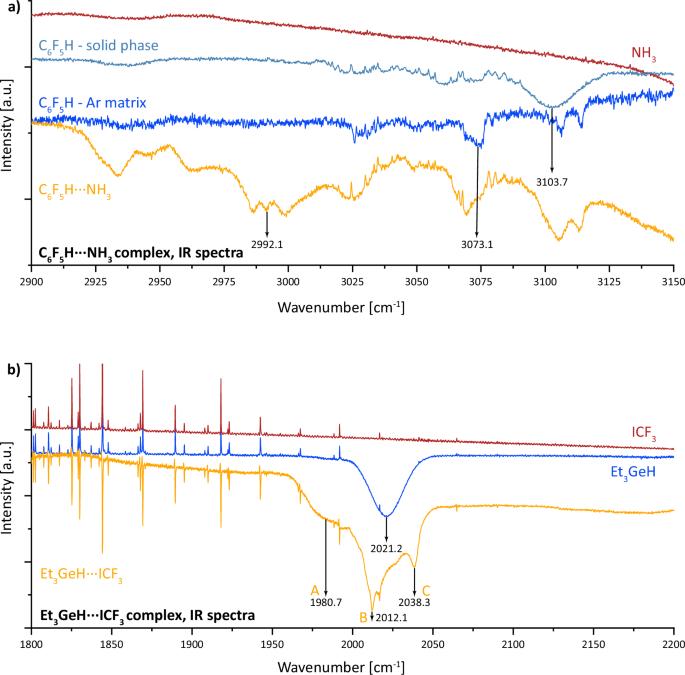
以前研究过的质子氢键和氢ridic 氢键配合物具有很大的相似性。本研究利用低温红外光谱和计算方法对质子氢键和氢ridic 氢键的结构、稳定性、电子特性和光谱特征进行了详细研究。利用实验和计算工具分析了五氟苯与氨(C₆F₅H⋯NH₃)和三乙基锗与三氟碘甲烷(Et₃GeH⋯ICF₃)的络合物。此外,还对 30 个具有质子氢键的络合物和 30 个具有氢ridic 氢键的络合物进行了计算研究。我们的研究结果表明,尽管这些氢键中氢的原子电荷相反,因此质子氢键和氢ridic 氢键中电子转移的方向也相反,但它们的光谱表现--特别是 X-H 伸展频率的红移和强度的增加--却非常相似。研究还讨论了目前国际理论化学和应用化学联合会(IUPAC)对氢键的定义在涵盖这两种类型氢键方面的局限性,并提出了克服这些局限性的方法。了解氢键对许多自然科学领域都至关重要。在本文中,作者利用低温红外光谱和计算方法研究了质子氢键和氢ridic 氢键,发现尽管这些氢键中的氢的原子电荷相反,但它们的光谱表现却非常相似。本文章由计算机程序翻译,如有差异,请以英文原文为准。
On the similar spectral manifestations of protonic and hydridic hydrogen bonds despite their different origin
Previously studied complexes with protonic and hydridic hydrogen bonds exhibit significant similarities. The present study provides a detailed investigation of the structure, stabilization, electronic properties, and spectral characteristics of protonic and hydridic hydrogen bonds using low-temperature infrared (IR) spectroscopy and computational methods. Complexes of pentafluorobenzene with ammonia (C₆F₅H⋯NH₃) and triethylgermane with trifluoroiodomethane (Et₃GeH⋯ICF₃) were analyzed using both experimental and computational tools. Additionally, 30 complexes with protonic hydrogen bonds and 30 complexes with hydridic hydrogen bonds were studied computationally. Our findings reveal that, despite the opposite atomic charges on the hydrogens in these hydrogen bonds, and consequently the opposite directions of electron transfer in protonic and hydridic hydrogen bonds, their spectral manifestations - specifically, the red shifts in the X–H stretching frequency and the increase in intensity - are remarkably similar. The study also discusses the limitations of the current IUPAC definition of hydrogen bonding in covering both types of H-bonds and suggests a way to overcome these limitations. Understanding hydrogen-bonding is essential for many fields of natural science. Here, the authors investigate protonic and hydridic hydrogen bonds using low-temperature infrared spectroscopy and computational methods, finding that despite opposite atomic charges on the hydrogens in these hydrogen bonds their spectral manifestations are remarkably similar.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


