高锰酸钾和 N-羟基琥珀酰亚胺引发的茚的自由基链有氧氧化作用
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了在 KMnO4 和 N-hydroxysuccinimide (NHSI) 的存在下,分子氧对积雪烯 (RH) 的液相氧化动力学。结果表明,加入醋酸后反应速率会增加。研究发现 KMnO4 和 NHSI 对 RH 的有氧氧化具有协同作用。这可能与 C-centered (R⦁) 和 O-centered (SINO⦁) 自由基的形成有关,C-centered 自由基参与了链的起始阶段,而 SINO⦁ 自由基则催化了链的扩展阶段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
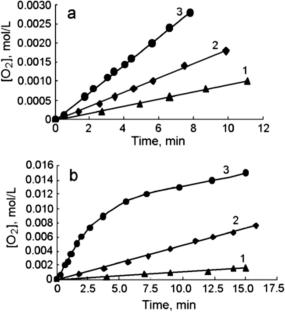
Initiation of Radical-Chain Aerobic Oxidation of Cumene by Potassium Permanganate and N-Hydroxysuccinimide
The kinetics of the liquid-phase oxidation of cumene (RH) with molecular oxygen in the presence of KMnO4 and N-hydroxysuccinimide (NHSI) is studied. An increase in the reaction rate when adding acetic acid is established. An synergistic effect of KMnO4 and NHSI in aerobic oxidation of RH is found. This can be associated with the formation of C-centered (R⦁) and O-centered (SINO⦁) radicals that participate in the chain initiation stage and the catalysis of the chain propagation stage by the SINO⦁ radical.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


