发现治疗新生隐球菌和白色念珠菌感染的新型真菌 Jumonji H3K27 去甲基化酶抑制剂
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
侵袭性真菌感染已成为一个严重的公共卫生问题。为了应对抗真菌治疗疗效有限和严重耐药性的挑战,迫切需要具有新作用机制的抗真菌药物。我们之前的研究发现,JIB-04 是真菌组蛋白去甲基化酶(HDM)的抑制剂。为了促进靶点验证和抑制剂设计,我们在此设计并合成了一系列新的 JIB-04 衍生物。在建立了结构-活性关系后,化合物 A4 被确认对新型隐球菌和白色念珠菌具有强效抗真菌活性。与先导化合物 JIB-04 相比,化合物 A4 是一种更有效的 HDM 抑制剂,具有更好的水溶性、毒力因子抑制活性和体内抗真菌效力。总之,这项研究进一步证实了真菌 HDMs 是潜在的抗真菌靶标,而化合物 A4 则是一种很有前途的抗真菌先导化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
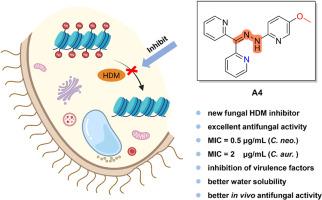
Discovery of new fungal jumonji H3K27 demethylase inhibitors for the treatment of Cryptococcus neoformans and Candida auris infections
Invasive fungal infections have become a serious public health problem. To tackle the challenges of limited efficacy in antifungal therapy and severe drug resistance, antifungal drugs with new mechanisms of action are urgently needed. Our previous study identified JIB-04 to be an inhibitor of fungal histone demethylase (HDM). To promote target validation and inhibitor design, herein a series of new JIB-04 derivatives were designed and synthesized. After the establishment of structure-activity relationship, compound A4 was identified to possess potent antifungal activity against Cryptococcus neoformans and Candida auris. Compared to lead compound JIB-04, compound A4 was a more potent HDM inhibitor and exhibited better water solubility, virulence factors inhibitory activity and in vivo antifungal potency. Collectively, this study further confirmed that fungal HDMs were potential antifungal targets and compound A4 was a promising antifungal lead compound.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


