内向和外向质子泵蛋白质子转运方向不同的原因
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
主动转运是生物现象中无处不在的重要过程。离子泵视网膜素是光驱动的活性离子转运体,具有七螺旋跨膜结构支架,其中全反式视网膜发色团通过希夫碱与第七跨膜螺旋中的保守赖氨酸残基共价键合。来自盐卤杆菌(Halobacterium salinarum)的细菌视紫红质是第一个被发现的离子泵视紫红质,并被鉴定为质子外向泵视紫红质。自 1971 年发现细菌视紫红质以来,又从跨越三个领域(细菌、古生菌和真核生物)的各种微生物和巨型病毒中分离出了更多的离子泵视紫红质。除了质子泵视蛋白外,还发现了氯离子和钠离子泵视蛋白。此外,人们还发现了离子泵浦型视蛋白在离子转运方向上的多样性,即最近发现了向内泵浦质子的视蛋白。非常有趣的是,内向质子泵视蛋白与外向质子泵视蛋白具有相同的结构特征和许多保守的关键残基。然而,尽管种类越来越多,但一个核心问题仍然没有改变:离子泵视网膜蛋白如何以及为什么会发生连锁构象变化,从而在蛋白质内部实现单向离子转移?在这方面,比较内向和外向质子泵视蛋白质子泵过程中的结构及其演变是一种有效的策略,因为通过比较可以发现质子单向传输的关键因素。我们通过时间分辨共振拉曼光谱阐明了质子泵入型和质子泵出型斜视蛋白的质子泵入机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
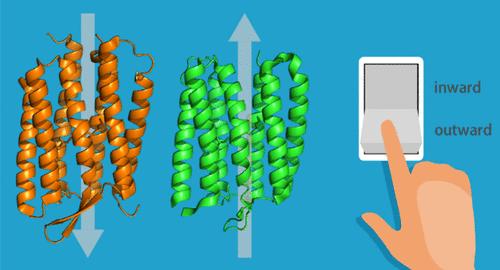
Origin of the Difference in Proton Transport Direction between Inward and Outward Proton-Pumping Rhodopsins
Active transport is a vital and ubiquitous process in biological phenomena. Ion-pumping rhodopsins are light-driven active ion transporters that share a heptahelical transmembrane structural scaffold in which the all-trans retinal chromophore is covalently bonded through a Schiff base to a conserved lysine residue in the seventh transmembrane helix. Bacteriorhodopsin from Halobacterium salinarum was the first ion-pumping rhodopsin to be discovered and was identified as an outward proton-pumping rhodopsin. Since the discovery of bacteriorhodopsin in 1971, many more ion-pumping rhodopsins have been isolated from diverse microorganisms spanning three domains (bacteria, archaea, and eukaryotes) and giant viruses. In addition to proton-pumping rhodopsins, chloride ion- and sodium ion-pumping rhodopsins have also been discovered. Furthermore, diversity of ion-pumping rhodopsins was found in the direction of ion transport; i.e., rhodopsins that pump protons inward have recently been discovered. Very intriguingly, the inward proton-pumping rhodopsins share structural features and many conserved key residues with the outward proton-pumping rhodopsins. However, a central question remains unchanged despite the increasing variety: how and why do the ion-pumping rhodopsins undergo interlocking conformational changes that allow unidirectional ion transfer within proteins? In this regard, it is an effective strategy to compare the structures and their evolutions in the proton-pumping processes of both inward and outward proton-pumping rhodopsins because the comparison sheds light on key elements for the unidirectional proton transport. We elucidated the proton-pumping mechanism of the inward and outward proton-pumping rhodopsins by time-resolved resonance Raman spectroscopy, a powerful technique for tracking the structural evolutions of proteins at work that are otherwise inaccessible.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


