Rab1a 的增加通过激活 mTORC1-S6K 通路抑制自噬,从而加速骨关节炎的发生
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
导言由于软骨细胞代谢平衡失调,软骨降解是骨关节炎(OA)进展过程中的关键性改变。自噬在维持细胞内平衡方面发挥着重要作用。最近的研究越来越多地强调了自噬在调节 OA 潜在病理机制中的重要性。本研究旨在阐明Rab1a是否能通过调节软骨细胞自噬和凋亡来调控OA的发生。对基因本体论术语和京都基因组百科全书通路进行了严格鉴定。通过qPCR、Western印迹和免疫荧光等方法探讨了Rab1a的作用以及Rab1a、mTORC1、自噬和细胞凋亡之间的相互作用。为了证实 Rab1a 在体内 OA 发病机制中的作用,研究人员还建立了小鼠 OA 实验模型。结果Rab1a在炎性软骨细胞和膝关节OA软骨中的表达明显上调。抑制 Rab1a 可部分缓解体外和体内细胞外基质降解和细胞凋亡,而过表达 Rab1a 则会加剧软骨基质降解和细胞凋亡。此外,还观察到 Rab1a 水平升高会抑制自噬并激活 mTORC1-S6K 信号通路,从而加重 OA 的发病机制。这一发现表明,Rab1a 是预防和治疗 OA 的一个前景广阔的创新治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
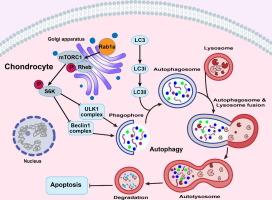
Increased Rab1a accelerates osteoarthritis by inhibiting autophagy via activation of the mTORC1-S6K pathway
Introduction
Cartilage degradation is a critical alteration in the progression of osteoarthritis (OA) due to the disorder of chondrocyte metabolic homeostasis. Autophagy plays an important role in maintaining intracellular homeostasis. Recent investigations have increasingly underscored the importance of autophagy in modulating the pathological mechanisms underlying OA. Ras-related protein Rab-1a (Rab1a) has been illustrated to regulate autophagy in many diseases but not in OA.Objectives
This study aims to elucidate whether Rab1a could regulate the development of OA through modulation of chondrocyte autophagy and apoptosis.Methods
Proteomic sequencing, Western blotting, and immunohistochemistry were applied to detect the expression level of Rab1a in vitro and in vivo. Gene Ontology terms and Kyoto Encyclopedia of Genes and Genomes pathways were rigorously identified. The effects of Rab1a and the interaction between Rab1a, mTORC1, autophagy and apoptosis were explored by qPCR, Western blotting, and immunofluorescence. An experimental mouse OA model was also performed to confirm the role of Rab1a in OA pathogenesis in vivo. Histological analysis was employed to demonstrate cartilage damage.Results
Rab1a expression was significantly upregulated in inflamed chondrocytes and knee OA cartilage. Inhibition of Rab1a partially attenuated the degradation of the extracellular matrix and cell apoptosis both in vitro and in vivo, whereas overexpression of Rab1a intensified cartilage matrix degradation and cellular apoptosis. Additionally, elevated Rab1a levels were observed to suppress autophagy and activate the mTORC1-S6K signaling pathway, thereby aggravating OA pathogenesis.Conclusion
The augmentation of Rab1a expression impairs autophagy and promotes apoptosis through the activation of the mTORC1-S6K signaling pathway, further exacerbating OA pathogenesis. This finding suggests that Rab1a serves as a promising and innovative therapeutic target for the prevention and treatment of OA.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


