精胺介导 RIPK1 乙酰化,抑制糖尿病的发生和发展
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
已经证实,N-乙酰转移酶(小鼠 NAT1(mNAT1)和人类 NAT2(hNAT2))介导 2 型糖尿病患者的胰岛素敏感性。在这里,我们发现 mNAT1 的缺乏会导致细胞精胺的减少,而精胺是一种天然多胺,具有保护健康和抗衰老的作用,但人们对其机制的了解还很有限。我们发现,mNAT1 和 hNAT2 能够调节受体丝氨酸/苏氨酸蛋白激酶 1(RIPK1)--炎症和细胞死亡的关键调节因子--上皮苷乙酰化的一种翻译后修饰,我们将其命名为乙酰化上皮苷。补充精胺可减少 RIPK1 介导的细胞死亡以及 NAT1 缺乏在体内诱发的糖尿病表型。此外,抑制 RIPK1 可以阻止 NAT1 缺乏小鼠血管病理学介导的胰岛素抵抗和糖尿病肾病。最后,我们证明了人类糖尿病患者血管组织中精胺的减少和 RIPK1 的激活。我们的研究表明,血管病理学在糖尿病的发病和进展中扮演着重要角色,并确定了抑制 RIPK1 激酶是治疗 2 型糖尿病的一种潜在治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
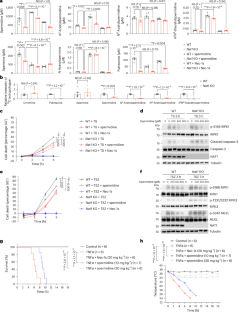

Spermidine mediates acetylhypusination of RIPK1 to suppress diabetes onset and progression
It has been established that N-acetyltransferase (murine NAT1 (mNAT1) and human NAT2 (hNAT2)) mediates insulin sensitivity in type 2 diabetes. Here we show that mNAT1 deficiency leads to a decrease in cellular spermidine—a natural polyamine exhibiting health-protective and anti-ageing effects—but understanding of its mechanism is limited. We identify that mNAT1 and hNAT2 modulate a type of post-translational modification involving acetylated spermidine, which we name acetylhypusination, on receptor-interacting serine/threonine-protein kinase 1 (RIPK1)—a key regulator of inflammation and cell death. Spermidine supplementation decreases RIPK1-mediated cell death and diabetic phenotypes induced by NAT1 deficiency in vivo. Furthermore, insulin resistance and diabetic kidney disease mediated by vascular pathology in NAT1-deficient mice can be blocked by inhibiting RIPK1. Finally, we demonstrate a decrease in spermidine and activation of RIPK1 in the vascular tissues of human patients with diabetes. Our study suggests a role for vascular pathology in diabetes onset and progression and identifies the inhibition of RIPK1 kinase as a potential therapeutic approach for the treatment of type 2 diabetes. Zhang et al. show that the activation of RIPK1 is suppressed by acetylhypusination in a spermidine-dependent manner. Disruption of this axis contributes to RIPK1-mediated vascular pathology to promote insulin resistance and diabetic kidney pathology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


