铂基合金催化剂在氨氧化反应中的反应途径和抗中毒机理
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在电化学氨氧化反应(AOR)中,合金化策略是提高铂基催化剂活性的有效方法。尽管如此,其反应途径和抗中毒机理仍有待详细研究,以加深理解。本研究同时考虑了 OS 和 GM 机制,通过密度泛函理论系统地研究了铂和铂锰合金(M = Fe、Co、Ni、Cu 和 Zn)的反应途径和抗中毒机理。观察到 PtFe、PtCo、PtCu 和 PtZn 合金的形成增强了 AOR 的动力学。对于铂、铂钴和铂锌来说,由于反应能垒比 OS 机制低,GM 机制在 AOR 过程中更为普遍。值得注意的是,这项研究表明,Fe、Co、Cu 和 Zn 的引入引起了铂原子电子结构的变化,导致铂-N 键的弱化,促进了 *N 和 *N2 的解吸,因此分别增强了 OS 和 GM 机制的抗中毒特性。该研究加深了对铂基合金催化剂反应途径和抗中毒机理的理解,为设计高效稳定的 AOR 催化剂提供了有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
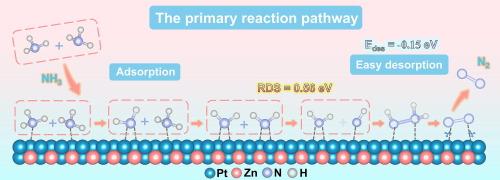
Reaction pathway and anti-poisoning mechanism of Pt-based alloy catalysts in the ammonia oxidation reaction
In the electrochemical ammonia oxidation reaction (AOR), the alloying strategy is an effective method to improve the activity of Pt-based catalysts. Nevertheless, its reaction pathway and anti-poisoning mechanisms still need to be studied in detail to improve the understanding. In this study, the OS and GM mechanisms are considered simultaneously to systematically investigate the reaction pathway and anti-poisoning mechanism of Pt and PtM alloys (M = Fe, Co, Ni, Cu, and Zn) by density functional theory. The formation of PtFe, PtCo, PtCu, and PtZn alloys was observed to enhance the kinetics of the AOR. For Pt, PtCo, and PtZn, the GM mechanism is more prevalent in the AOR process due to the lower reaction energy barrier compared to the OS mechanism. Notably, this work demonstrates that the introduction of Fe, Co, Cu, and Zn induces an electronic structure change of Pt atoms, resulting in the weakening of the Pt−N bond and facilitating the desorption of *N and *N2, therefore enhancing the anti-poisoning properties of the OS and GM mechanisms, respectively. This study deepens the understanding of the reaction pathway and anti-poisoning mechanisms of Pt-based alloy catalysts and presents an effective strategy for designing efficient and stable AOR catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


