记忆 B 细胞在系统性红斑狼疮发病机制中的重要作用
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
新的证据表明,记忆B细胞在系统性红斑狼疮(SLE)中功能失调。它们对通过 B 细胞受体(BCR)发出的信号反应迟钝,但对 Toll 样受体(TLR)和 I 型干扰素信号以及通过 CD40-CD154 激活 T 细胞介导的信号仍有反应。长期暴露于核糖核蛋白(RNP)特异性自身抗体和 TLR 抗原或 BCR 抗原的免疫复合物可能会导致这种部分过敏表型。TLR7或TLR8信号和由此产生的I型干扰素,以及旁观者T细胞的持续激活,在记忆B细胞中形成了一个正向前馈循环,可以逃避负向选择,允许抗RNP自身抗体优先扩增。自体干细胞移植或B细胞靶向单克隆抗体和嵌合抗原受体(CAR)T细胞的临床试验表明,记忆B细胞群的补充与系统性红斑狼疮的复发有关。此外,记忆性B细胞对BCR的低反应性可能是非消耗性B细胞靶向疗法(包括BTK抑制剂和抗CD22单克隆抗体)在系统性红斑狼疮中失败的原因。因此,靶向功能失调的记忆B细胞可能会被证明对系统性红斑狼疮有效,同时还能避免广谱靶向不直接参与疾病发病机制的B细胞和浆细胞亚群的不良反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
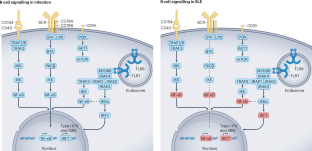
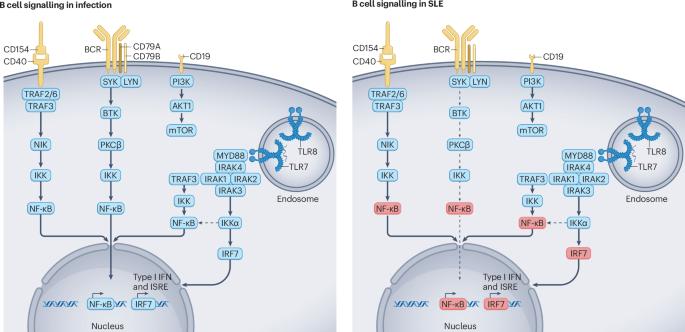
The essential roles of memory B cells in the pathogenesis of systemic lupus erythematosus
Emerging evidence indicates that memory B cells are dysfunctional in systemic lupus erythematosus (SLE). They are hyporesponsive to signalling through the B cell receptor (BCR) but retain responsiveness to Toll-like receptor (TLR) and type I interferon signalling, as well as to T cell-mediated activation via CD40–CD154. Chronic exposure to immune complexes of ribonucleoprotein (RNP)-specific autoantibodies and TLR-engaging or BCR-engaging cargo is likely to contribute to this partially anergic phenotype. TLR7 or TLR8 signalling and the resulting production of type I interferon, as well as the sustained activation by bystander T cells, fuel a positive feedforward loop in memory B cells that can evade negative selection and permit preferential expansion of anti-RNP autoantibodies. Clinical trials of autologous stem cell transplantation or of B cell-targeted monoclonal antibodies and chimeric antigen receptor (CAR) T cells have correlated replenishment of the memory B cell population with relapse of SLE. Moreover, the BCR hyporesponsiveness of memory B cells might explain the failure of non-depleting B cell-targeting approaches in SLE, including BTK inhibitors and anti-CD22 monoclonal antibodies. Thus, targeting of dysfunctional memory B cells might prove effective in SLE, while also avoiding the adverse events of broad-spectrum targeting of B cell and plasma cell subsets that are not directly involved in disease pathogenesis. BCR-independent memory B cell reactivation via TLR7 or TLR8 activation, type I interferon production, immune complex formation and T helper cell signalling is central in SLE pathogenesis. Dörner and Lipsky discuss the potential of targeting these pathways to eliminate autoreactive memory B cells and plasma cells in SLE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


