利用煅烧和未煅烧的镁锌铁三元层状双氢氧化物(LDH)高效去除水溶液中的刚果红
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究利用煅烧和未煅烧的[Mg0.52+Zn0.252+Fe0.253+(OH)2].(CO32-)0.125.H2O 三元层状双氢氧化物(LDH)研究了从水溶液中去除纺织工业染料刚果红的问题。XRD 证实了 LDH 晶体结构的成功形成。傅立叶变换红外光谱(FTIR)分析表明,1108 cm-1 处出现了峰值,这表明存在 S = O 基团。TEM 分析显示形成了六角形的形态,而 BET 测量则表明煅烧后表面积有所改善。本研究分析了染料吸附受相互作用时间、溶液 pH 值和吸附剂用量的影响。吸附数据采用 Langmuir 等温线和 Freundlich 等温线进行分析,动力学数据则采用伪一阶、伪二阶和颗粒内扩散模型进行分析。焓的变化(ΔH0)为正值,吉布斯自由能的变化(ΔG0)为负值,说明吸附过程是自发的内热过程。朗缪尔等温线模型分析了吸附等温线数据,并计算出吸附剂吸附的吸附剂量。煅烧和未煅烧的层状双氢氧化物对刚果红的最大吸附量分别为 205.76 和 89.76 mg g-1。这些结果表明,煅烧过的层状双氢氧化物是一种去除废水中废水的重要吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
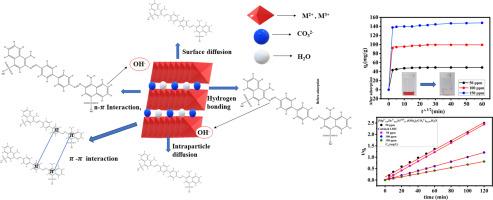
Efficient removal of Congo red from aqueous solutions using calcined and uncalcined MgZnFe ternary layered double hydroxide (LDH)
This study investigates the textile industries' dye Congo-red removal from aqueous solution using the calcined and uncalcined ternary layered double hydroxide (LDH). XRD confirms the successful formation of the crystalline structure of LDH. FTIR analysis shows that the peak position at 1108 cm−1, which indicates the presence of S = O group. TEM analysis reveals the formation of a hexagonal shape morphology, whereas BET measurements demonstrate an improvement in surface area after calcination. This study analyzed dye adsorption, which is affected by interaction time, solution pH, and adsorbent dosage. The adsorption data is analyzed using the Langmuir isotherm and Freundlich isotherm, while the kinetics data is analyzed using pseudo-first-order, pseudo-second-order, and intraparticle diffusion models. The change in enthalpy (ΔH0) being positive and the change in Gibbs free energy (ΔG0) being negative designates that the adsorption process is endothermic and occurs spontaneously. The Langmuir isotherm model analyzed the adsorption isotherm data and calculated the amount of adsorbate adsorbed by adsorbent. The maximum amount of Congo-red adsorbed by calcined and uncalcined layered double hydroxide are 205.76 and 89.76 mg g-1. These results suggested that calcined layered double hydroxide is a significant adsorbent for removing effluents from wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


