三种具有五个 N-杂环的多功能二氟硼荧光染料,可用于机械氟变色行为、无墨书写和潜在指纹成像
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-11-01
DOI:10.1016/j.jphotochem.2024.116125
引用次数: 0
摘要
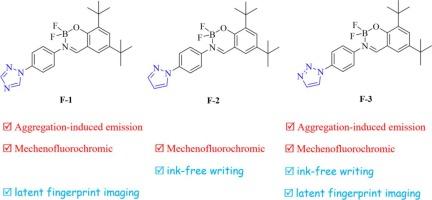
为了进一步推动多功能荧光染料的开发,研究人员合成了三种二氟硼化合物(F-1、F-2 和 F-3),它们含有五元 N-杂环分子作为电子吸收单元。结果表明,含有 1,2,4- 三氮唑部分的化合物 F-1 和含有 1,2,3- 三氮唑部分的化合物 F-3 具有适合潜指纹成像的聚集诱导发射活性。相比之下,含有吡唑单元的化合物 F-2 则表现出聚集引起的淬灭现象。此外,这三种化合物都显示出机械氟变色行为。具体来说,固态化合物 F-1 的发射光谱在研磨时仅发生约 5 纳米的轻微偏移,而化合物 F-2 则发生了从 475 纳米到 501 纳米的红色偏移,化合物 F-3 则从 482 纳米偏移到 507 纳米。此外,化合物 F-2 和 F-3 都可用于无墨书写。值得注意的是,使用化合物 F-2 制备的安全纸因其不可逆的机械氟变色行为而设计为一次性使用,相反,由化合物 F-3 制备的安全纸因其可逆的机械氟变色行为而可重复书写。这为探索具有 1,2,3-三唑分子的多功能二氟硼化合物的应用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Three multifunctional difluoroboron fluorescent dyes with five member N-heterocyclic ring for mechanofluorochromic behaviors, the ink-free writing and latent fingerprints imaging
To further advance the development of multifunctional fluorescent dyes, three difluoroboron compounds (F-1, F-2, and F-3) containing five-membered N-heterocyclic ring moieties that act as the electron-withdrawing units were synthesized. It was observed that compound F-1, which incorporated a 1,2,4-triazole segment, and compound F-3 with 1,2,3-triazole portion exhibited aggregation-induced emission activities suitable for latent fingerprints imaging. In contrast, compound F-2 featuring a pyrazole unit demonstrated an aggregation-caused quenching phenomenon. Additionally, all three compounds displayed mechanofluorochromic behaviors. Specifically, the emission spectra of compound F-1 in the solid state experienced only a slight shift of approximately 5 nm upon grinding, however, compound F-2 underwent a red shift from 475 to 501 nm while compound F-3 shifted from 482 to 507 nm. Furthermore, both compounds F-2 and F-3 could be utilized in ink-free writing applications. Notably, the safety paper prepared using compound F-2 was designed for single-use due to its irreversible mechanofluorochromic behavior, conversely, the safety paper derived from compound F-3 was rewritable owing to its reversible mechanofluorochromic behavior. It provided a foundation for exploration the application of multifunctional difluoroboron compounds with 1,2,3-triazole moiety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


