基于后修饰 Zr-MOF 的开启式荧光传感器,用于对映体选择性识别苯丙氨酸
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
手性对映体,尤其是氨基酸,经常显示出不同的生理活性和生物功能。因此,区分它们的绝对构象至关重要。在这里,通过缩合反应将手性中心 l-脯氨酸(L-Pro)和 d-脯氨酸(D-Pro)固定到 Zr 基金属有机框架(MOF)中,得到了一对手性传感器 UiO-L-Pro 和 UiO-D-Pro。荧光分析表明,UiO-L-Pro 和 UiO-D-Pro 在用 l-苯丙氨酸(L-Phe)或 d-苯丙氨酸(D-Phe)处理时,其荧光强度的增强程度存在明显差异,这证明了它们具有对映选择性发光特性。UiO-L-Pro 和 UiO-D-Pro 的对映选择性系数 α(α = KBH(D-Phe)/KBH(L-Phe))分别为 4.15 和 0.47。因此,手性材料可用于鉴定苯丙氨酸的不同构型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
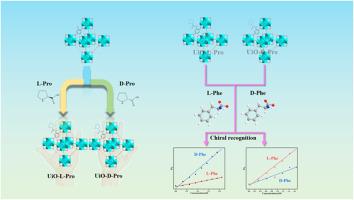
Turn-on fluorescent sensors based on post-modified Zr-MOF for enantioselective recognition of phenylalanine
Chiral enantiomers, particularly amino acids, frequently display distinct physiological activities and biological functions. Consequently, it is crucial to distinguish their absolute conformations. Herein, a pair of chiral sensors, UiO-L-Pro and UiO-D-Pro, were obtained by immobilizing the chiral center l-proline (L-Pro) and d-proline (D-Pro) into a Zr-based metal−organic framework (MOF) via a condensation reaction. Fluorescence analyses revealed a notable difference in the enhancement of fluorescence intensity between UiO-L-Pro and UiO-D-Pro when treated with l-phenylalanine (L-Phe) or d-phenylalanine (D-Phe), demonstrating enantioselective luminescence properties. Differences based on hydrogen bond interaction give them significant enantioselectivity factors α. The enantioselectivity factors α (α = KBH(D-Phe)/KBH(L-Phe)) for UiO-L-Pro and UiO-D-Pro were 4.15 and 0.47, respectively. Thus, the chiral material could be employed to identify different configurations of phenylalanine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


