具有内置电场的 MOF 衍生 NiCo2S4/FeS 异质结构用于增强淡水/盐水/乙醇/甲醇中的电氧化作用
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
氧进化反应(OER)作为水分解的半反应,具有很高的理论过电位。因此,开发具有高 OER 性能的电催化剂有利于电解制氢。在导电基底上原位生长纳米材料是制备电催化剂的有效策略。在这项工作中,我们在碳布基底上生长了 Ni/Co 双金属金属有机框架 (MOF),并通过 MOF 衍生策略成功构建了一种坚固的 NiCo2S4/FeS@CC 电催化剂。这种电催化剂可用于实现高效、稳定的 OER 性能。MOF 衍生的 NiCo2S4/FeS 具有多孔异质结构和多组分的优点,活性位点多,电荷转移速率快,而硫掺杂则大大提高了 OER 性能。这种自支撑异质材料在碱性淡水中的电流密度高达 10 mA cm-2,过电位仅为 238 mV。NiCo2S4/FeS@CC 在碱性盐水中表现出良好的 OER 性能,10 mA cm-2 时过电位仅为 241 mV。我们推测这是由于在电氧化过程中催化剂表面生成了带负电荷的 SO42- 阴离子层,从而有效地避免了 Cl- 的腐蚀。这两种条件都表现出了极佳的长期稳定性。此外,我们还通过在电解液中添加乙醇或甲醇,成功地进一步降低了起始电位和能耗。这项工作为提高 MOF 衍生过渡金属电催化剂的 OER 性能提供了一种有效的方法,可用于电解水制氢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
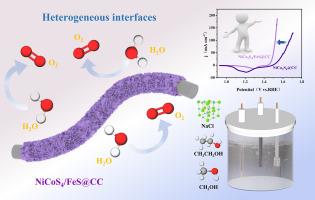
MOF-derived NiCo2S4/FeS heterostructures with built-in electric field for enhanced electrooxidation in freshwater/ brine /ethanol/methanol
Oxygen evolution reaction (OER), as a half-reaction of water decomposition, has a high theoretical overpotential. Therefore, the development of electrocatalysts with high OER performance is favorable for electrolytic hydrogen production. In situ growth of nanomaterials on conductive substrates is an effective strategy for the preparation of electrocatalysts. In this work, we grew Ni/Co bimetallic metal-organic framework (MOF) on carbon cloth substrates and successfully constructed a robust NiCo2S4/FeS@CC electrocatalyst through a MOF derivatization strategy. This electrocatalyst can be used for efficient and robust OER performance. MOF-derived NiCo2S4/FeS has the advantage of a porous heterostructure and multicomponent with many active sites and faster charge transfer rate, while sulfur doping greatly improves the OER performance. The current density of this self-supported heterogeneous material in alkaline freshwater reaches up to 10 mA cm−2 with an overpotential of only 238 mV. NiCo2S4/FeS@CC exhibited good OER performance in alkaline brine with an overpotential of only 241 mV at 10 mA cm−2. We speculate that this is due to the generation of a negatively charged SO42− anionic layer on the catalyst surface during the electrooxidation process, which effectively avoids Cl− corrosion. And both conditions demonstrate excellent long time stability. Additionally, we succeeded in further reducing the onset potential and energy consumption by adding ethanol or methanol to the electrolyte. This work provides an effective method to improve the OER performance of MOF-derived transition metal electrocatalysts for hydrogen production from electrolytic water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


