直接口服抗凝剂对慢性肾病的安全性和疗效:荟萃分析
IF 3.4
3区 医学
Q2 HEMATOLOGY
Research and Practice in Thrombosis and Haemostasis
Pub Date : 2024-10-01
DOI:10.1016/j.rpth.2024.102584
引用次数: 0
摘要
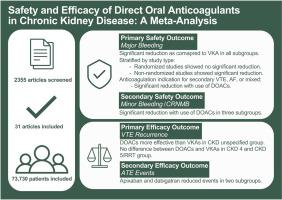
背景直接口服抗凝剂(DOAC)已成为心房颤动患者预防静脉血栓栓塞和中风的一线疗法。方法我们检索了从开始到 2023 年 6 月的 MEDLINE 和 Embase,以查找在 CKD 患者中比较 DOAC 与维生素 K 拮抗剂 (VKAs) 的随机和非随机队列研究。筛选和数据收集一式两份。主要安全性结果为大出血,根据国际血栓与止血学会标准进行定义,并按 CKD 严重程度进行分层。结果在文献检索获得的 2355 篇文章中,纳入了 25 项非随机研究(n = 6832)和 6 项随机研究(n = 66898)。与 VKAs 相比,DOACs 可减少所有亚组的大出血(4 期:OR,0.73;95% CI,0.58,0.93;5 期/肾脏替代治疗:OR,0.70;95% CI,0.50,0.98;未指定分期:OR,0.72;95% CI,0.63,0.83)。阿哌沙班和利伐沙班都能减少第 5 期/肾脏替代治疗患者的大出血(阿哌沙班:OR,0.66;95% CI,0.63;利伐沙班:OR,0.72;95% CI,0.63):OR,0.66;95% CI,0.52,0.85;利伐沙班:结论在这项荟萃分析中,与 VKAs 相比,DOACs 可减少 4 期、5 期/肾替代治疗和 CKD 分期未定患者的大出血。未来的分析应评估特定 DOACs 和剂量对该人群安全性和有效性的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Safety and efficacy of direct oral anticoagulants in chronic kidney disease: a meta-analysis
Background
Direct oral anticoagulants (DOACs) have emerged as the first-line therapy for venous thromboembolism and stroke prophylaxis in atrial fibrillation. As DOACs are partially excreted renally, their safety in patients with chronic kidney disease (CKD) is unclear.
Objectives
To synthesize primary evidence on the safety profile of DOACs in patients with CKD.
Methods
We searched MEDLINE and Embase from inception to June 2023 for randomized and nonrandomized cohort studies comparing DOACs with vitamin K antagonists (VKAs) in CKD patients. Screening and data collection were conducted in duplicate. The primary safety outcome was major bleeding, defined by International Society on Thrombosis and Haemostasis criteria, stratified by CKD severity. Meta-analysis was done using the Mantel–Haenszel random-effects model, presented as odds ratios (ORs) with corresponding 95% CIs.
Results
Of the 2355 articles captured in the literature search, 25 nonrandomized studies (n = 6832) and 6 randomized studies (n = 66,898) were included. DOACs reduced major bleeding compared with VKAs in all subgroups (stage 4: OR, 0.73; 95% CI, 0.58, 0.93; stage 5/renal replacement therapy: OR, 0.70; 95% CI, 0.50, 0.98; stage unspecified: OR, 0.72; 95% CI, 0.63, 0.83). Apixaban and rivaroxaban both reduced major bleeding in stage 5/renal replacement therapy patients (apixaban: OR, 0.66; 95% CI, 0.52, 0.85; rivaroxaban: OR, 0.58; 95% CI, 0.35, 0.94).
Conclusion
In this meta-analysis, DOACs reduced major bleeding compared with VKAs in stage 4, stage 5/renal replacement therapy, and CKD stage unspecified patients. Future analysis should evaluate the impact of specific DOACs and dosage on safety and efficacy in this population.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Research and Practice in Thrombosis and Haemostasis
Medicine-Hematology
CiteScore
5.60
自引率
13.00%
发文量
212
审稿时长
7 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


