大气成核前体对甲酸硫酐诱导成核的增强作用:理论机制。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
作为 H2SO4(SA)形成的中间体,甲酸硫酐(FSA)被假定在大气气溶胶的成核过程中发挥作用。这是第一次从理论上系统地研究了簇合物 (SA)x(A)y(W)n 和 (FSA)x(A)y(W)n (x=1-2;y=1-2;n=0-4)的结构、热力学、分子间相互作用、湿度依赖性、大气依赖性和光学特性。预测 FSA 比 SA 更能促进氨(A)的成团,这表明取代基增强了 FSA 的成核能力。而取代基并不影响水合团簇对湿度的敏感性。水合簇有形成小水合簇(nwater≦3)的趋势。对大气依赖性的研究表明,团簇的稳定性更多地取决于温度而不是压力。此外,与 A、SA 和水分子相比,FSA 在降低大气能见度方面表现出更强的能力。这一发现旨在引起人们对 FSA 在大气中成核的关注。本文章由计算机程序翻译,如有差异,请以英文原文为准。
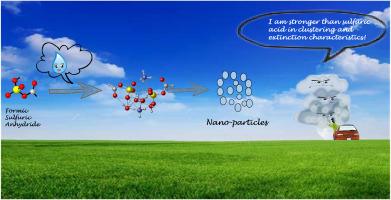
Enhancement of atmospheric nucleation precursors on formic sulfuric anhydride induced nucleation: Theoretical mechanism
As an intermediate formed by H2SO4 (SA), formic sulfate anhydride (FSA) has been hypothesized to play a role in the nucleation of atmospheric aerosols. It is the first time that the clusters (SA)x(A)y(W)n and (FSA)x(A)y(W)n (x = 1–2; y = 1–2; n = 0–4) were systematically studied in theory on the structures, thermodynamics, intermolecular interactions, humidity dependence, atmospheric dependence and optical properties. FSA is predicted to be more stronger to promote the clustering with ammonia (A) than SA, suggesting that substituent group enhances nucleation capability of FSA. Whereas, the substituent group does not influence the humidity sensitivity of hydrated clusters. The clusters trend to form small hydrated clusters (nwater≦3). The study on atmospheric dependence indicates that the stability of the clusters depends more on temperature other than pressure. Moreover, FSA shows a stronger ability on reducing atmospheric visibility than A, SA and water molecules. This finding aims to draw attention to FSA about atmospheric nucleation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


